Common
Identified Coral Diseases
Aspergillosis-Disease
Overview
Aspergillosis is a lesion producing fungal infection of
Caribbean soft corals. It affects 6 sp. of sea fans and sea whips and is
widespread throughout the Caribbean. The pathogen is Aspergillosis sydowii, a terrestrial
fungus (Geiser et al., 1996), which infects gorgonia after germination of
spores on the coral surface. This is followed
by penetration and spread of hyphae in coral tissue, resulting in highly
visible lesions.
Lesions may be associated with complete loss of tissue and
skeleton, and often occur at multiple sites across an infected colony. Purple
galls may be produced by the coral host to encapsulate fungal hyphae. If such
galls are present, fungal hyphae are visible if the gall is cut open. One
known reservoir is African dust. See (Alker et al., 2001; Geiser et
al., 1998; Kim and Harvell, 2002; Kim et al., 2000; Nagelkerken et al.,
1997a,b; Smith et al., 1996, 1998.)
Infected
colony of Gorgonia ventalina
Aspergillosis is caused by the
terrestrial fungus Aspergillus sydowi. The disease results in lesions
associated with degraded gorgonian tissue. Gorgonia counteract the disease by
encapsulating fungal hyphae in purple pigmented galls.
Bacterial
Bleaching-Disease Overview
Bleaching caused by a
specific bacterial infection (as opposed to a response to environmental stress)
occurs when loss of zooxanthellae is due to a toxin produced by the
intracellular bacterial pathogen. Bacterial bleaching occurs in the Mediterranean
scleractinian coral Oculina patagonica. Two known bleaching pathogens are Vibrio shiloi and V. patogonica
(Kushmaro et al., 2001, in press) . Initial pathogen attachment to coral
is specific to a b-galactose containing receptor in the coral host surface
mucopolysaccharide layer. Subsequent invasion of coral host tissue is
followed by temperature dependent intracellular growth of bacteria.
Production of a heat sensitive toxin results in lysis of the
zooxanthellae. Disease signs consist of loss of zooxanthellae, with coral
tissue intact, and differs from environmental bleaching in that Vibrio
shiloi or patogonica are
present in the affected tissue. The reservoir is not known. (See
Kushmaro et al., 1996, 1998; Ben-Haim et al., 1999; Banin et al., 2000, 2001,
in press.)
Bacterial Bleaching

Oculina patagonica partially bleached colony (in the
field)
Bacterial bleaching is
caused by a specific bacterial/coral interaction. Specificity includes
recognition by the pathogen of host (coral) surface receptors; invasion of
coral tissue; multiplication of bacteria in coral tissue; and release of
bacterial toxins that cause bleaching.
Black Band -Disease
Overview
Black band disease is
characterized by complete coral tissue degradation due to a pathogenic microbial
consortium that appears as a dark red or black migrating microbial mat. The mat is present between apparently
healthy coral tissue and freshly exposed coral skeleton. The band color may be blackish brown to red
depending on the vertical position of a cyanobacterial population associated
with the band. The vertical position is
based on a light intensity-dependent photic response of the cyanobacterial
filaments, and the color (due to the cyanobacterial pigment phycoerythrin) is
dependent on the thickness of the band.
The band is approximately 1 mm thick and ranges in width from 1 mm to 7
cm. White specks may be present on
surface, at times forming dense white patches. The pathogenic microbial mat consortium moves across coral
colonies at rates from 3 mm to 1 cm/day.
Tissue death is caused by exposure to an anoxic, sulfide-rich
microenvironment associated with the base of the band.
The black band microbial
consortium consists of an assortment of photosynthetic and non-photosynthetic
bacteria that co-exist synergistically.
The consortium has three functionally and physically dominant members as
well as numerous heterotrophic members whose role in the disease is as yet
unknown. The three functionally
dominant members are populations of cyanobacteria, sulfate-reducing, and
sulfide-oxidizing bacteria. The black
band disease microbial consortium is structurally and functionally identical to
cyanobacterial-dominated microbial mats found in other illuminated,
sulfide-rich environments (Carlton and Richardson, 1995; Richardson et al.,
1997) .
Several species of
cyanobacteria have been found associated with black band disease (Frias-Lopez
et al.,2002; Cooney et al., 2002), the
most well-known of which is Phormidium
corallyticum (Rützler and Santavy, 1983) .
Sulfide-oxidizing bacteria, dominated by Beggiatoa spp. (Ducklow and
Mitchell, 1979) , are present in well-developed bands and exhibit visible
vertical migrations within the band matrix (Richardson, 1996; Viehman and
Richardson, in press). When present on
the band surface Beggiatoa appears white due to intracellular
inclusions of stored elemental sulfur.
Sulfate-reducing bacteria dominated by Desulfovibrio spp. (Ducklow
and Mitchell, 1979; Frias-Lopez et al.,2002;
Cooney et al., 2002) are present at the base of the band and are
responsible for producing high concentrations of sulfide within the band matrix
(Carlton and Richardson, 1995). Light
microscopic observation of black band reveals motile (gliding) filaments of P. corallyticum that are 4 mm wide, with one round end and one
narrow (sharply tapering) end. Also
present are gliding Beggiatoa
filaments (1-4 mm wide) that are non-pigmented but contain highly
refractive intracellular granules of elemental sulfur. Numerous gram negative
bacteria (small rods) are also present but not identifiable using light
microscopy. The bacterial population
has been characterized using molecular techniques and was found to contain over
500 species of bacteria that are different from bacterial communities found in
the water column, healthy coral tissue, or dead coral skeleton (Frias-Lopez et
al., 2002; Cooney et al., 2002). The functional role of this diverse
population of bacteria is not known.
Black band disease
affects 42 species of coral in a worldwide distribution. The only known reservoir is within
cyanobacterial biofilms that are
present on sediments in depressions of healthy black band disease
susceptible corals (Richardson, 1997) .
Black Band Disease
Black band disease is
characterized by a dark ring, or band, that separates apparently healthy coral
tissue from freshly exposed coral skeleton. It migrates across coral
colonies completely degrading coral tissue. A closeup view reveals that
the band is composed of numerous microorganisms, here revealed as a dark
community of phosynthetic cyanobacteria (“bluegreen algae”) and white specks of
sulfur bacteria.
*** Click images to
view full sized high resolution image ***
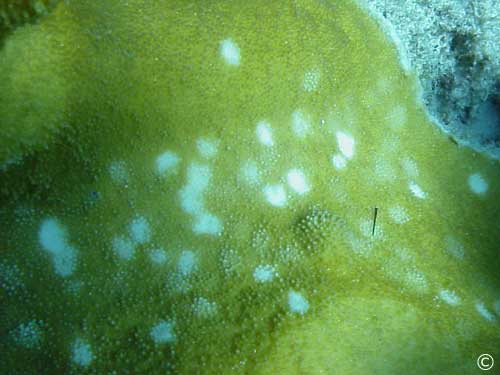
Dark Spots -Disease
Overview
Dark spots disease is
present as dark (brown or purple) pigmented areas of tissue on scleractinian
corals. There is no known pathogen. The
pigmented areas may or may not overly recessed areas of coral skeleton. The coral tissue remains intact, although at
times lesions and coral tissue death are observed in the centers of the
spots. ( Gil-Agudelo and
Garzon-Ferreira, 2001) . This disease
is widespread throughout the Caribbean.
Infected Colony

Dark Spots-S.intersepta

Dark Spots-Sid
There is no known
pathogen for dark spots disease, which is recognized by darkly pigmented
patches on coral tissue. Tissue loss is minimal, if present.
White Band -Disease
Overview
White band disease is
characterized by complete coral tissue degradation of Caribbean acroporid
corals. Two species are affected, Acropora palmata and A. cervicornis (Gladfelter,
1982). The disease exhibits a sharp
demarcation between apparently healthy coral tissue and exposed coral
skeleton. These signs are identical to
plague, except that white band is acroporid specific (and plague has not been
found on acroporids). Tissue loss
usually proceeds from the base of the
colony branch to the tip, although it can begin in the middle of a branch in A. cervicornis.
There are two distinct disease
types that differ in the pattern of tissue loss. White band Type I exhibits tissue degradation associated with a
line that migrates across the coral colony.
There is no obvious microbial band, although the freshly exposed coral
skeleton appears band like. Tissue
lysis is always associated with the moving front (which differentiates Type I
from Type II. The rate of tissue loss
varies from mm to cm/day (Peters et al., 1983). White band Type II also exhibits tissue degradation as a band
moves across a coral colony, however in this case the moving front may, at
times, have bleached zone that catches up to active tissue lysis (Ritchie and Smith, 1998) .
The only way to distinguish the two types is to observe the band
progression over time.
No known pathogen has been
isolated (and has only been attempted for type II), although there is a
documented shift in the composition of the population of bacteria present in
the surface mucopolysaccharide layer.
The shift is from domination by psuedomonads to domination by Vibrio carchariae (Ritchie and Smith, 1995). Histopathological examination of white band
Type I diseased tissue may reveal aggregates of gram negative bacteria in
affected tissue (Peters et al., 1983) .
White band disease affects
acroporids throughout the Caribbean and has decimated populations at a regional
scale (Gladfelter, 1982; Peters et al. 1983; Aronson and Precht, 1997, 2001).
Infected Colony

White Band type II in
Acropora cervicornis
There are two etiologies of white band disease, type I and
type II. In type I, tissue destruction is associated with the moving
front of the band. In type II, there is at times a bleached zone between
the area of tissue degradation and the moving front. If the bleached zone
is not present, type I is visually indistinguishable from type II. No pathogen
has been isolated.
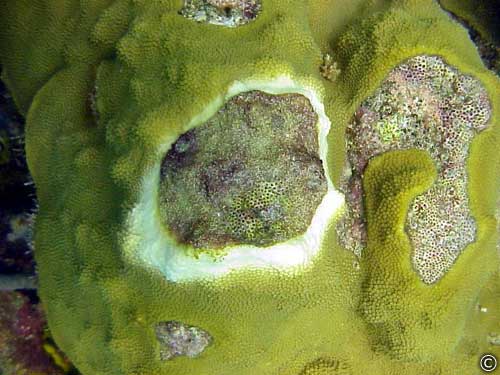
White Plague -Disease
Overview
Plague is characterized by a sharp
line between apparently healthy coral tissue and freshly exposed coral
skeleton. There is no obvious microbial
band present. Plague is caused by the
bacterial pathogen Aurantimonas
coralicida, gen nov. sp. nov. (Denner
et al., IJSEM, in press). Disease signs (rate and pattern of
disease progression and virulence) vary between three distinct types. Plague
Type I, documented in the 1970s and 1980s,
starts at the sides of colonies, with tissue destruction at a rate of 3
mm/day. Six species were reported to be
affected (Dustan, 1977, Dustan and Halas, 1987). Plague Type II, first documented in 1995, starts at the base of a
coral colony and progresses upward, with tissue destruction up to 2
cm/day. A bleached zone between healthy
tissue and exposed skeleton (<3 mm) may be present (Richardson et al.
1998a,b). Plague type III, first seenin
1999, starts on the sides or top of colonies and destroys tissue at high rates
of dm/day. It is found on massive Montastraea annularis and Colpophyllia natans. Plague is currently epidemic throughout
the Caribbean, and affects 33 sp. of Caribbean scleractinian corals (Weil et
al, in press). The reservoir is not known.
Plague (synonym: white
plague)
White Pox -Disease
Overview
White
pox is characterized by coral tissue degradation that occurs in association
with circular lesions on the Caribbean
scleractinian coral Acropora
palmata. Rapid loss of tissue
progresses along a distinct line, or with small remnants of tissue sometimes
present near the margin of, irregularly shaped patches anywhere on the upper or
lower surfaces of Acropora palmata
branches. The average rate of tissue
loss is 2.5 cm2/day, although rates up to 10.5 cm2/day
can occur. It is caused by the
bacterium Serratia marcescens, a
well-known species that is widespread in both terrestrial and aquatic
environments as well as in mammalian and arthropod hosts (Patterson et al.,
2002).
White Pox (synonym: acroporid serratiosis)
White pox is characterized by circular lesions. The pathogen is Serratia marcescens, a gram negative member
of the enterobacteria.
*** Click images to view full sized high
resolution image ***
Yellow Band -Disease
Overview
Yellow band is
characterized by large rings or patches of bleached, yellow tissue on Caribbean
scleractinian corals. It affects Montastraea annularis and M. faveolata and is widespread throughout
the Caribbean. Tissue loss is
extremely slow (cm/year). When yellow band infected corals are also bleached
the yellow band can blend in with bleaching signs; after recovery from
bleaching the band becomes visible again.
No pathogen has been discovered, although loss of zooxanthellae pigments
and zooxanthellae cells in affected tissue have been documented (Cervino et
al., 2001). Yellow band associated
zooxanthellae have lower mitotic
indices (number of dividing cells), and it has been suggested that this disease
affects the zooxanthellae and not the coral (Cervino et al., 2001).
Yellow Band
Yellow band
is characterized by a bleached zone that expands in a halo. Tissue loss is
minimal (cm/yr). No pathogen has been isolated.
Yellow
band on Montastraea faveolata
Information on this page is courtesy NOAA, go
to http://www.coral.noaa.gov/coral_disease/
for more up-to-date and detailed information