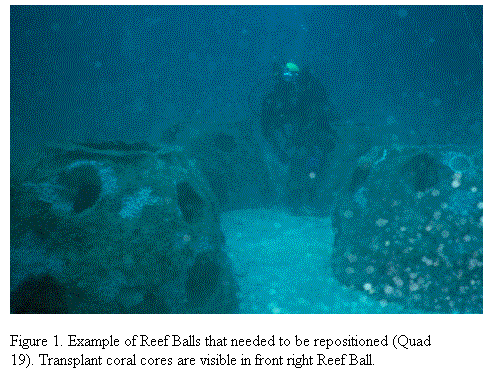Memphis Project Annual Report:
July 2002 – July 2003
Submitted to:
Broward
County Department of Planning and Environmental Protection
218 SW First Avenue
Fort Lauderdale, Florida 33301
Prepared by:
Richard E. Spieler, Ph.D. and
T. Patrick Quinn, M.S.,
Oceanographic Center
Nova Southeastern University
8000 North Ocean Drive
Davie, Florida 33004
Submitted by:
_________________________
Date: _________________
Richard E. Spieler, Ph.D.
The United States nuclear
submarine MEMPHIS grounded in approximately 10 m of water on a southeastern
Florida coral reef off Broward County in February 1993. This grounding caused
extensive physical and biological damage to the reef substrate and to the coral
community. As part of a mitigation plan, in July 2000 a three-year experimental
restoration project was initiated. This is a report of the third year of that
project. However, the annual reports are cumulative in text, data and analyses
and therefore this report also contains information from the first two year’s
work.
In order to gain insight into
optimal methodology for restoring corals to a damage site, the project compares
settlement, growth, and survival rate of corals amongst artificial reefs
treated with potential attractants (iron, quarry rock, coral transplants) and
no-attractant controls. Further, in order to examine appropriate structural
design for restoration, the reefs are divided into four treatments of
structural complexity. This allows the determination of the interactive effects
of four different fish communities on coral settlement and growth. In addition,
the work investigates the potential role of microbial biofilms as settlement
precursors. The transplant treatments are identical replicates (same numbers of
each species). This will allow the determination of species specific
differential survival and growth rates of coral transplants. Finally, the four
complexity treatments will yield insight into fish community restoration
methodology (the hypothesis here is that multiple refuge size is required for a
diverse coral reef community).
The experimental design consists
of 160 small (1.13 m) Reef Ballsä organized into 40, 4-module reef units (quads) each in
a square configuration with 3-m sides. Each quad has Reef Balls with the four
attractant treatments, one Reef Ball per attractant (iron, quarry rock, coral
transplants, or only concrete). Each Reef Ball has two standardized settlement
plates incorporating the attractant treatment of the Reef Ball. The 40 quads
are divided into four different levels of structural complexity. One set of 10
quads has the void spaces of all the Reef Balls empty. One set has the void
spaces of all filled with structure offering small refuge (plastic caging).
Another set of 10 has the void spaces of all filled with large refuge (concrete
block). The final set of 10 quads is mixed and has one Reef Ball empty, one
with large refuge, and the last two with small refuge.
One hundred and sixty Reef Balls,
433 settlement plates, and 1500 biofilm discs were constructed July-August
2000. The Reef Balls were deployed in November 2000. However, many Reef Balls were not deployed on designated
sites and extensive delay to the research was incurred due to the time needed
to adjust the positions. The final arrangement was achieved in June 2001. Settlement plates and biofilm discs were coated with
concrete and attractants (iron filings, calcium carbonate sand) or concrete
without attractants (controls) in July 2001 and were deployed in August 2001. Quarterly data collection
was initiated in October 2001. In excess of 670 individual SCUBA dives have
been made to date.
The biofilm discs were removed at
1, 3, 7, 14, 21 and 60 day intervals and examined microscopically for
biofouling. The control discs and those incorporating the calcium carbonate did
not differ in the settlement rate of bacteria or diatoms. However, the discs
with iron filings had a significantly slower settling rate than both the
calcium carbonate and control discs.
In August 2002, a study was also
initiated to examine the potential for a red coralline alga (Hydrolithon boergesenii) to act as an attractant for coral settlement on
restoration concrete structure. Settlement plates were formed into tent shaped
modules by the addition of a concrete base and deployed at four sites, eight
modules per site. One site was on a field of coral rubble with abundant H. boergesenii. A second, nearby, site on a sand
field was selected as control, and cleaned of incidental algae coated rubble
for a 10 m radius. The third site was adjacent to hard bottom on a rubble field
with abundant H. boergesenii. Site four, the control for #3, was located on
hardbottom with abundant hard corals but which lacked H. boergesenii or abundant rubble. Our hypothesis is: if H. boergesenii provides a
coral attractant, more hard coral should recruit to plates surrounded by the
algae than in control areas without the presence of the algae. After 12 months
no coral recruits were macroscopically visible on settlement plates.
Caging
and concrete fill was added to the Reef Balls in May-July 2001 to acquire
differential complexity. As hypothesized, after 12 months, there were already
different fish assemblages associated with the differing fill. At 24 months,
for total abundance of fish, the empty reef balls did not differ from those
with mixed fill however both these treatments were significantly less than
either small or large fill which did not differ from each other. With species
richness, the empty reef balls had fewer species than those with small fill
which, in turn, had fewer than either mixed or large fill treatments which did
not differ from each other. An understanding of the potential interaction of
these differing assemblages with coral recruitment and mortality awaits
photographic analysis of the settlement plates.
Coral species were selected for
transplantation (Montastrea cavernosa and Meandrina
meandrites) and donor colonies located. The first four transplants were
drilled out of donor colonies using a hydraulic drill in March 2001. The holes in the donor corals were filled
with concrete plugs and before-and-after photographs were taken of each
site. The cores were then taken to the
Reef Ball site and epoxyed into the appropriate Reef Balls. The last of the 80
transplants were completed in July 2001. After 23 months, 100% of the M. cavernosa and 27.5% of the M. meandrites transplants maintained
their original tissue surface area or showed evidence of an increase in surface
area. The remaining 72.5% of the M. meandrites transplants have shown
varying degrees of partial tissue mortality. The donor colonies have
experienced 100% colony survival. Although there has been some growth onto the
plugs, none of the core hole sites have regenerated tissue over an entire
concrete plug. However, there has been little tissue die back from the plug
sites and so complete regeneration remains probable. Clearly, the
species-specific differences in transplant growth and mortality, noted in this
study, indicate that species selection must be an important consideration in
future coral reef restoration efforts.
EXECUTIVE
SUMMARY................................................................................. 2
TABLE OF
CONTENTS..................................................................................... 4
INTRODUCTION.................................................................................................... 5
METHODS AND MATERIALS...................................................................... 5
Experimental Design............................................................................... 5
Reef Ball Construction and Deployment.................................................. 6
Coral Transplantation............................................................................. 7
Complexity Fill...................................................................................... 8
Coral Attractants.................................................................................... 8
Biofilm Research................................................................................... 8
Red Algae as Coral Attractant Study....................................................... 9
RESULTS................................................................................................................... 10
Coral Transplants................................................................................. 10
Biofilm................................................................................................ 12
Fish Assemblages................................................................................ 13
Settlement Plates.................................................................................. 13
Red Algae as Coral
Attractant Study..................................................... 13
DISCUSSION........................................................................................................... 14
APPENDIX............................................................................................................... 15
Memphis Project Timeline.................................................................... 16
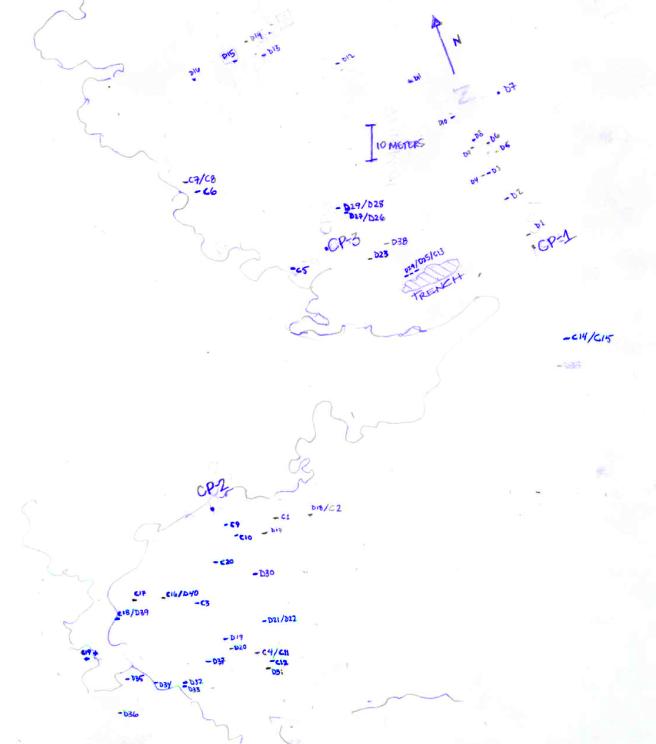
Final DGPS coordinates of the Reef Ball Quads.................................... 17
Map of Second Reef Depicting Site of Donor and Control Corals.......... 18
Memphis Restoration, 1st Quarterly Report........................................... 19
Memphis Restoration, 2nd Quarterly Report......................................... 19
Memphis Restoration, 3rd Quarterly Report.......................................... 21
Memphis Restoration, 4th Quarterly Report.......................................... 25
Memphis Restoration, 5th Quarterly Report.......................................... 30
Memphis Restoration, 6th Quarterly Report.......................................... 34
Memphis Restoration, 7th Quarterly Report.......................................... 36
Memphis Restoration, 8th Quarterly Report.......................................... 38
Memphis Restoration,
9th Quarterly Report.......................................... 41
Memphis Restoration,
10th Quarterly Report......................................... 43
Memphis Restoration,
11th Quarterly Report......................................... 44
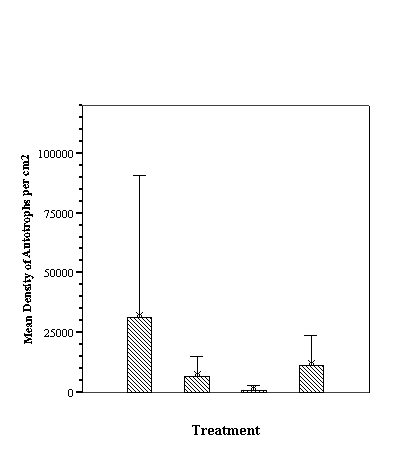
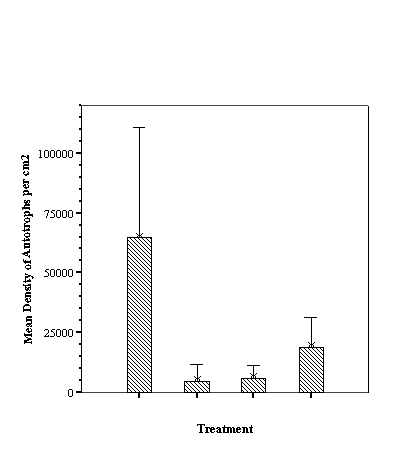
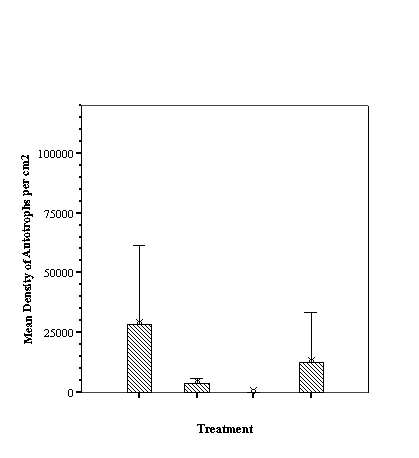
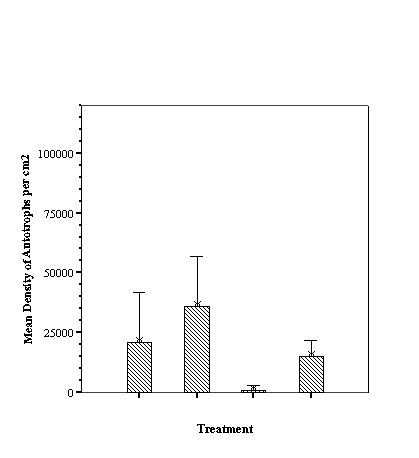
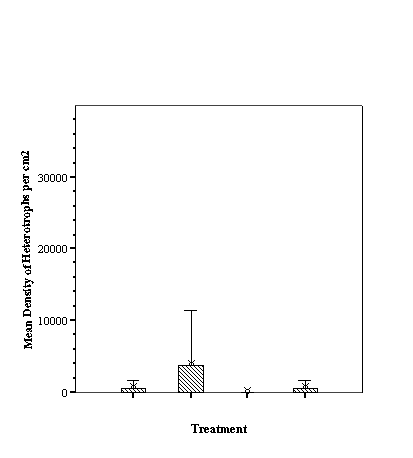
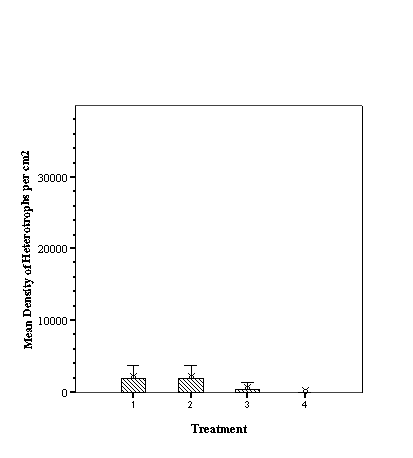
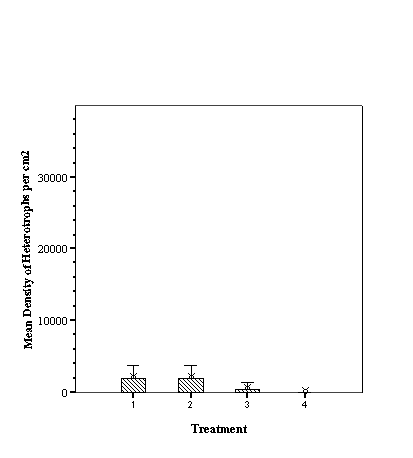
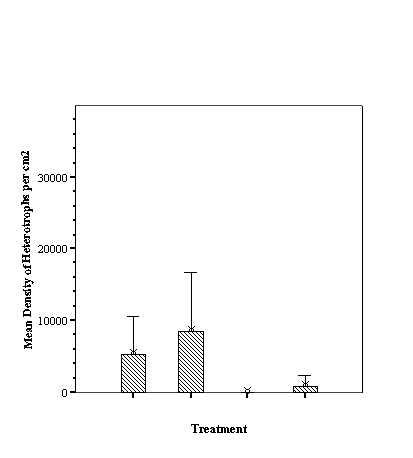
Descriptive Statistics: Species Richness Per Treatment.......................... 46
Descriptive Statistics: Fish Abundance Per Treatment............................ 47
Analysis of the Initial Microfouling Communities................................... 48
Scientific Presentations......................................................................... 75
The United States nuclear
submarine MEMPHIS grounded in approximately 10 m of water on a southeastern
Florida coral reef off Broward County in February 1993. This grounding caused
extensive physical and biological damage to the reef substrate and to the coral
community. As part of a mitigation plan, in July 2000 a three-year experimental
restoration project was initiated. However, because of technical difficulties
that delayed the initiation of data collection for almost a year, it was
decided to continue the study for an additional 12 months. This is a report of
the third year of the project. However, the annual reports are cumulative in
text, data and analyses and therefore this report also contains information
from the first two year’s work. A breakdown of the annual task accomplishment
is provided in the project timeline and the quarterly reports (see Appendix).
In order to gain insight into
optimal methodology for restoring corals to a damage site, the project compares
settlement, growth, and survival rate of corals amongst artificial reefs
treated with potential attractants (iron, quarry rock, transplants, and
no-attractant controls). Further, in order to examine appropriate structural
design for restoration, the reefs are divided into four treatments of
structural complexity. This allows the determination of the interactive effects
of four different fish communities on coral settlement and growth. The
transplant treatments are identical replicates (same numbers of each species).
This will allow the determination of species specific differential survival and
growth rates of coral transplants. Finally, the four complexity treatments will
yield insight into fish community restoration methodology (the hypothesis here
is that multiple refuge size is required for a diverse coral reef community).
In addition, two sub-studies investigate the potential role of microbial
biofilms as settlement precursors and the potential of a coralline red algae (Hydrolithon boergesenii) to act as an attractant to restoration-concrete
structure.
The experimental design consists
of 160 small (1.13 m) Reef Ballsä organized into 40, 4-module reef units (quads) each in
a square configuration with 3-m sides. Each quad has Reef Balls with the
four-attractant treatments, one Reef Ball per attractant (iron, quarry rock,
coral transplants, or only concrete). Each Reef Ball has two standardized
settlement plates incorporating the attractant treatment of the Reef Ball. The
40 quads are divided into four different levels of structural complexity. One
set of 10 quads has the void spaces of all the Reef Balls empty. One set has the
void spaces of all filled with structure offering small refuge (plastic
caging). Another set of 10 has the void spaces of all filled with large refuge
(concrete block). The final set of 10 quads is mixed and has one Reef Ball
empty, one with large refuge, and the last two with small refuge. In addition,
biofilm discs were attached on Reef Balls adjacent the main study area. These
discs were removed at 1, 3, 7, 14, 21 and 60-day intervals and examined for
biofouling.
Construction of Reef Balls
began on July 31, 2000 and was completed August 18, 2000. The construction
involved a total of 494 labor hours and concluded with the completion of 168
Reef Balls (suitable for deployment), 433 settlement plates and 1500 biofilm
discs. The settlement plates and biofilm discs were constructed at this time to
ensure the concrete mixture was the same for Reef Balls and attractant
structures. Approximately 40 additional
Reef Balls were constructed that were rejected due to flaws. The Reef Balls and
settlement plates were stored at Nova Southeastern University’s Oceanographic
Center (NSUOC) until deployment.
On September 12, 2000 NSUOC
personnel surveyed the deployment site to map the reef edge and search for hard
bottom between the reefs. Deployment of
the Reef Balls took place on November 17, 2000. The first quad (group of four
Reef Balls) was deployed at approximately 0700 hrs and the 40th quad
was deployed at approximately 1420 hrs.
NSUOC personnel attempted to
map the grid of quads from November 27, 2000 to January 4, 2001 using SCUBA
divers with slates. After six dives, the Reef Balls did not appear to be in a
recognizable pattern and eight quads could not be found. To obtain a more
detailed map and find the missing quads, NSUOC personnel chose a day (January
6, 2001) with very good surface-to-bottom visibility and live-boated over the
grid area. DGPS coordinates were
recorded each time the boat passed over a quad. The coordinates were then
entered into a program written specifically for the purpose of charting the
grid. Divers then swam the grid with a
laminated hard-copy of the chart and signaled the boat each time they were at a
quad. The signal was to submerge the dive flag and then hold the flag directly
over the quad. The boat came up to the flag, paused and the DGPS coordinate was
recorded. This was accomplished on January 11-12, 2001 and the missing eight
quads were found. The coordinates were reentered into the charting program to
acquire an accurate map of the grid area.
It was determined that approximately 16 quads would need to be
repositioned to approach the desired 30-meter separation. In addition, a number
of individual Reef Balls within quads were not spaced correctly. NSUOC
personnel started repositioning the Reef Balls on February 21, 2001. This
involved 2-3 divers and 4-5 100 lb. lift bags for each Reef Ball. The bags were attached to a Reef Ball,
inflated and the divers maneuvered the Reef Ball to obtain the correct spacing.
This work was completed on February 21, 2001; however an additional two Reef
Balls were later found that required correct spacing (Figure 1). Broward County
DPEP and NSUOC personnel started moving quads for the desired 30 meter
separation distance on March 3, 2001 and completed the task on June 5, 2001.
Approximately 29 dives were made to move 80 Reef Balls. There were two sets of quads that still did
not have the full 30-meter separation distance but it was decided to take this
into account, if necessary, in statistical evaluations and proceed with the
study. Each quad is labeled with a 3x5
inch plastic laminated tag containing the quad’s specific number. The DGPS
coordinates and label numbers are listed in the appendix.
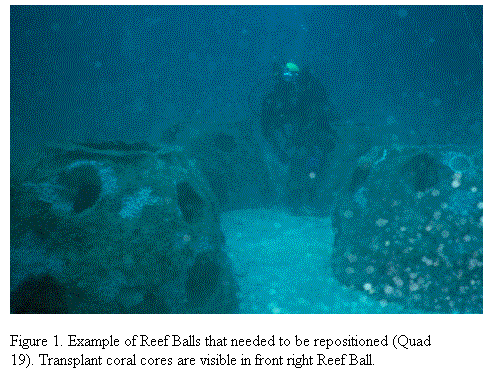
From January 26 to February 8,
2001, 89 concrete plugs were made to fill the holes in donor corals that would
be formed by removing the drilled transplant coral cores. On April 5, 2001 the
plugs were shortened to better fit the donor corals. The coral transplantation
work began on September 12, 2000 with a dive on the second reef, near the
Memphis grounding site, to assess the area for suitable species and numbers of
donor corals. Montastrea cavernosa
and Meandrina meandrites were
selected as transplant species due to availability and colony size. The first
four colonies were drilled using a Stanley Hydraulic drill on March 14,
2001. The holes in the donor corals
were filled with concrete plugs and before-and-after photographs were taken. The cores were then taken to the Reef Ball
site and epoxyed into the appropriate Reef Balls (Figure 2).

Corals for donors and controls
(non-drilled colonies) were mapped (see Appendix), tagged and photographed from
April 2, 2001 through June 11, 2001.
Requirements for controls were specimens of the appropriate size for
both donors and transplants. The trigger mechanism on the drill malfunctioned
in April and the drill was taken in for service. Technical problems continued
with the drill finally resulting in a new drill being sent to NSUOC on June 5,
2001. Drilling of donor corals resumed
on June 15, 2001 and was completed on July 6, 2001. Coral cores were placed in the appropriate Reef Balls the day
each core was drilled. The cores were photographed and secured into the Reef
Balls with epoxy from June 19 to June 24, 2001. Donor corals were photographed
and plugged from June 24 to July 10, 2001. Approximately 61 dives were made to
setup the coral transplantation and monitoring aspects of the project.
Photographs, taken at quarterly
intervals, are being used to determine coral growth over the course of the
study. The first complete monitoring session for 2001 was June-July. Monitoring consisted of a photographic
(slide) image of each study coral.
Photographic images of the transplants, the core hole sites (in the
donor corals), and the small controls are recorded using a Nikonos V camera
with a 28 mm lens and close up kit.
Photographic images of the entire donor colonies and the large control
colonies are recorded using a Nikonos V camera and a 20 mm lens with a 0.75 m2
PVC framer marked in 10 cm increments. The resulting slide images are scanned
using a Hewlett Packard Photosmart S20 slide scanner. SigmaScan Pro4 image analysis software (Jandel Scientific
Corporation) is being used for the analysis of the slide data. Individual slides were digitized then
calibrated using a ruler, included in the image, and measured in order to
determine tissue growth or retreat over time. This photographic technique has
allowed growth to be measured continually using an accurate and non-invasive
methodology. This technique is one of
the few monitoring methods in which the coral colony is not sacrificed and in
which changes in planar growth in two dimensions can be accurately assessed.
Plastic cage material and cinder
block were used for the small fill and large fill respectively. Approximately
50 m of plastic cage material (2 cm grid) were cut into triangular shapes,
rolled into cones and tie-wrapped into the Reef Balls by divers. The cinder
blocks were dropped overboard at the location of each quad assigned to have
large fill. Divers then collected the
block and placed them inside the Reef Balls. This work began on May 7, 2001 and
was completed on July 6, 2001.
Approximately 320 settlement
plates were coated with the appropriate attractants (iron granules, quarry
rock, or plain concrete) and cemented onto the Reef Balls in August 2001.
Approximately 1500 biofilm discs
were made during the Reef Ball’s construction of the same concrete mix. The
discs were coated with the appropriate attractants (iron granules, quarry rock,
or plain concrete) and stored at NSUOC.
The discs were attached in an array on extra Reef Balls deployed outside,
but adjacent to, the main study area in August 2001. A preliminary technique
study of biofilm attachment to the discs was accomplished in November 2001 and
the results were presented at American Society Limnology and Oceanography
Conference 2001 in Albuquerque, NM (Appendix). The biofilm discs were collected
over a two-month period and analyzed. Data analysis was completed July 2002. A
full discussion of the biofilm methods is included in an attached report
(Appendix).
A study was initiated to examine
the potential for using a red coralline alga (Hydrolithon boergesenii)
to enhance recruitment to restoration structure. Settlement plates, made in
July-August 2000 with the other settlement plates used on the Reef Balls, were
formed into tent shaped modules by the addition of a concrete base (Fig. 3) and
deployed July 18 and 19, 2002. The modules were placed offshore Broward County
at four sites, eight modules per site. One site was on a field of coral rubble
with abundant H. boergesenii. A second
nearby site on a sand field was selected as control, and cleaned of incidental
algae coated rubble for a 10 m radius. The third site was on a rubble field
adjacent to hard bottom. It also had abundant H. boergesenii, although on this site the algae
appeared to be predominately restricted to the underside of rubble pieces. Site
four, the control for #3, was located on the hardbottom with abundant hard
corals, but which lacked H. boergesenii or abundant rubble. Our
hypothesis is: if H. boergesenii provides a coral attractant,
more hard coral should recruit to plates surrounded by the algae than in
control areas without the presence of the algae. The settlement plates are
examined periodically for recruitment.

Figure 3. Example of settlement module.
After nine months, there was a highly significant difference
(p<0.01, G-test) between the two species of transplants in growth/mortality;
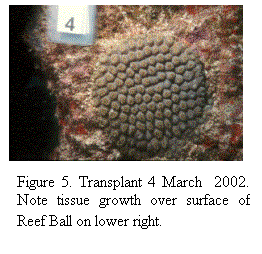
100% of the M. cavernosa (Fig. 4, 5)
and 71% of the M. meandrites
transplants maintained their original tissue surface area or showed evidence of
an increase in surface area. The
remaining 29% of the M. meandrites
transplants had shown varying degrees of partial tissue mortality.
Figure 4. Transplant 4,
March 2001.
|
|

|
|
Figure 6.
Transplant 4 on March 2003.
|
|
After
nine months, the donor colonies have experienced 100% colony survival. Although
the core hole sites had not regenerated tissue over the concrete plugs, there
had been little tissue die back from the plug sites, and a little growth (about
1%) in some cases. These data were presented at the International Society for
Reef Studies in September (Appendix).
After 23
months, 100% of the M. cavernosa and
27.5% of the M. meandrites
transplants maintained their original tissue surface area or showed evidence of
an increase in surface area (Fig. 6).
The remaining 72.5% of the M.
meandrites transplants have shown varying degrees of partial tissue
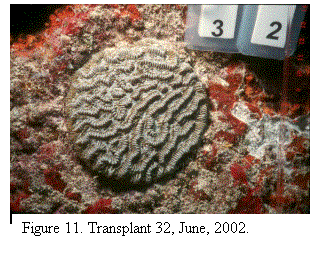
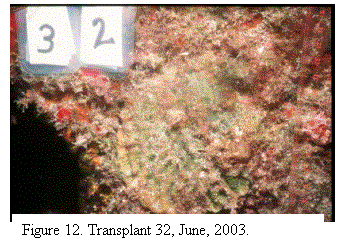
mortality (Fig. 7, 8, 9); 35% have died back completely (Fig. 10) and the
mortality remains ongoing (Fig.11, 12). The donor colonies continued to
experience 100% colony survival. Although there has been some measurable growth
(about 4%) onto some plugs, none of the core hole sites have regenerated tissue
over an entire concrete plug. However, there has been little tissue die back
from the plug sites and so complete regeneration remains probable.
Figure 9.
Transplant 57, June 2003.
|
|
Figure 8.
Transplant 57, June 2002.
|
|
Figure 7.
Transplant 57, July 2001.
|
|


|
|
Figure 10. Example of dead M. meandrites one year
post transplant.
|
|
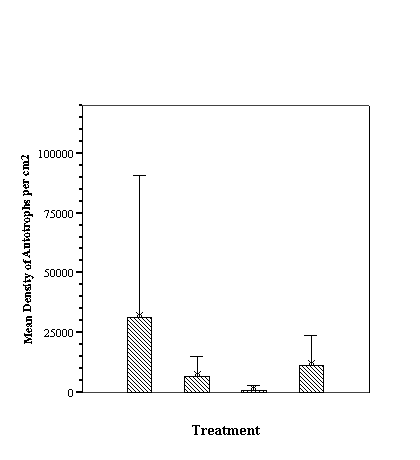
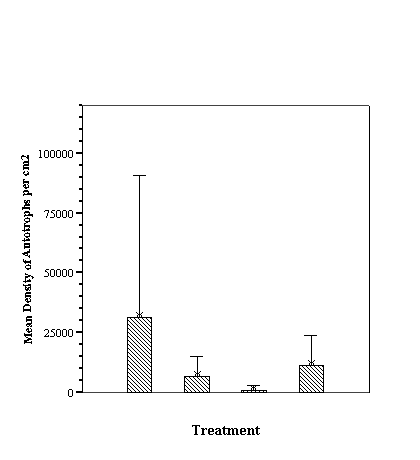
The biofilm study was completed
in 2002. The control discs and those incorporating the calcium carbonate did
not differ in the settlement rate of bacteria or diatoms. However, the discs
with iron filings had a significantly slower settling rate than both the
calcium carbonate and control discs (see Appendix for full report and complete
results).
As hypothesized there are
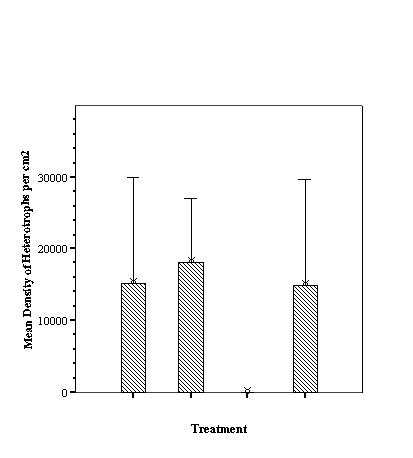
different fish assemblages associated with the differing fill. For total
abundance of fish the empty reef balls did not differ from those with mixed
fill however both these treatments were significantly less than either small or
large fill (p<0.05, ANOVA, SNK) which did not differ from each other
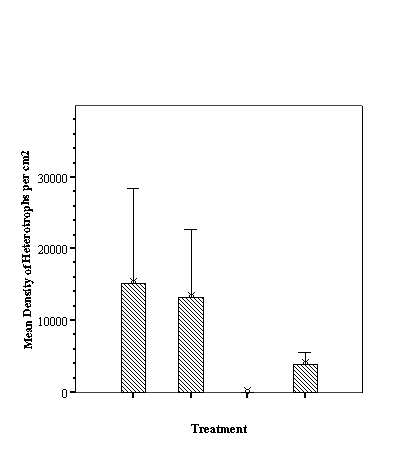
(p>0.05, SNK). With species richness, the empty reef balls had fewer species
than those with small fill which, in turn, had fewer than either mixed or large
fill treatments (p<0.05, ANOVA, SNK) which did not differ from each other
(p>0.05, SNK) (see Appendix).
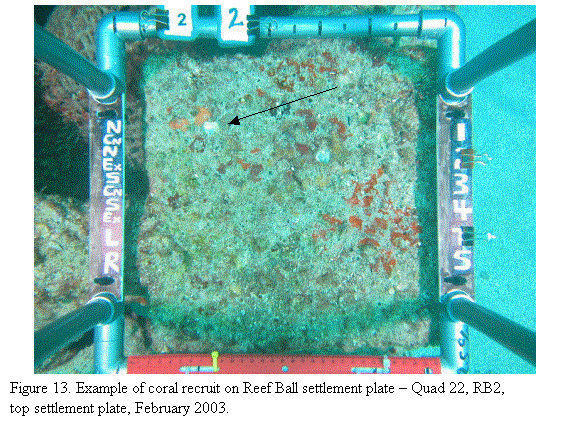
Several corals have recruited to
and grown on the Reef Ball attached settlement plates sufficient to allow ready
recognition as scleractinian corals (Fig. 13). However, additional growth is
required for more specific identification and additional recruitment is
required to provide adequate numbers for rigorous statistical evaluation among
settlement treatments. In the coming year, we intend to photograph and analyze
growth with the same methodology used with the transplanted corals.
Photos were taken of the
settlement plates six months after placement followed by a cursory macroscopic
reexamination after one year. No
noticeable coral recruits were recorded.
The similarity of the microbial
fouling of concrete and calcium carbonate coated biofilm discs is interesting.
Concrete leaching affecting the results can still not be entirely discounted,
as the thin layer of calcium carbonate sand may not have been sufficient to
reduce any leachate from the biofilm disc. The significant decrease in the rate
of fouling of the iron-coated discs is unexpected; possibly the filings were
oxidizing and sloughing off too rapidly for the biofilm to be maintained. Until additional results provide a clear
indication of coral settlement preferences, or lack thereof, the results of the
biofilm study are difficult to evaluate relative to their potential role in
coral recruitment.
The
difference in fish assemblages associated with the differing Reef Ball fill
treatments was anticipated. An understanding of the potential interaction of
these differing assemblages with coral recruitment and mortality awaits the
photographic analysis of the settlement plates. Likewise, the study on the
potential of H. boergesenii to
attract coral to restoration structure has only been underway for a year and
results of this study also await future photographic analysis of settlement
plates. For both the Reef Ball and H.
boergesenii studies there has been
insufficient coral recruitment to date to allow for rigorous analysis. For this
reason, we have extended the study duration for another 12 months. A final
study report will be provided to DPEP July 2004.
However, the data on coral
transplant does already provide some definitive results on species-specific
mortality. The cause(s) of the
mortality difference of the transplants is not clear. M.
meandrites donor corals were
apparently as resistant as M.
cavernosa to the stress of coring.
In addition, when entire colonies of M.
meandrites are transplanted either locally (Vernacchio
and Gilliam, unpublished data) or elsewhere (geology.uprm.edu/Morelock/GEOLOCN_/myzfinalRPT.htm), there is a low rate of mortality. Presumably the difference is due to
differential responses to some other aspect of the coring methodology or
perhaps a species-specific requirement for minimum transplant size. It has been
hypothesized (Kevin Helmle, NSUOC, personal communication) that the mortality
difference between the species may be due to differences in internal structure.
M. cavernosa colonies consist of discrete (plocoid)
monostomodaeal polyps. In contrast M. meandrites colonies are highly integrated (meandroid) and polystomodaeal. Thus, M. cavernosa may simply lose individual polyps to a gross injury, such as coring,
whereas a much larger portion of the M.
meandrites colony may be affected
and a small portion of the colony, such as a core, may not be able to repair
itself. If this is the case, similar differences in transplant mortality might
extend to other monostomodaeal (e.g., M. annularis complex) and
polystomodaeal (e.g. Diploria spp.) species. Obviously, more research is
needed for a causal determination. Nonetheless, the dramatic difference
in survival and growth between M.
cavernosa and M. meandrites transplants clearly
indicates that species selection and potential species-specific responses to
transplant methodology (e.g. coring) must be critical considerations in future
coral reef restoration efforts.
Memphis Project Timeline.................................................................... 16
Final DGPS coordinates of the Reef Ball Quads.................................... 17
Map of Second Reef Depicting Site of Donor and Control Corals.......... 18
Memphis Restoration, 1st Quarterly Report........................................... 19
Memphis Restoration, 2nd Quarterly Report......................................... 19
Memphis Restoration, 3rd Quarterly Report.......................................... 21
Memphis Restoration, 4th Quarterly Report.......................................... 25
Memphis Restoration, 5th Quarterly Report.......................................... 30
Memphis Restoration, 6th Quarterly Report.......................................... 34
Memphis Restoration, 7th Quarterly Report.......................................... 36
Memphis Restoration, 8th Quarterly Report.......................................... 38
Memphis Restoration,
9th Quarterly Report.......................................... 41
Memphis Restoration,
10th Quarterly Report......................................... 43
Memphis Restoration,
11th Quarterly Report......................................... 44
Descriptive Statistics: Species Richness Per Treatment.......................... 46
Descriptive Statistics: Fish Abundance Per Treatment............................ 47
Analysis of the Initial Microfouling Communities................................... 48
Scientific Presentations......................................................................... 75
Memphis
Project Timeline
|
Task
|
Scheduled Completion
|
Actual Completion
|
|
Reef Ball Construction
|
N/A
|
Aug. 2000
|
|
1st
Quarterly Report
|
Oct. 2000
|
Oct. 2000
|
|
Reef Ball Deployment
|
N/A
|
Nov. 2000
|
|
2nd
Quarterly Report
|
Jan. 2001
|
Jan. 2001
|
|
3rd
Quarterly Report
|
Apr. 2001
|
Apr. 2001
|
|
Final Reef Ball Positioning
|
N/A
|
June 2001
|
|
Coral Attachment and Assessment
|
N/A
|
June 2001
|
|
Reef Ball Complexity Fill
|
N/A
|
July 2001
|
|
4th
Quarterly Report
|
July 2001
|
July. 2001
|
|
1st Yearly Report
|
July 2001
|
July 2001
|
|
Biofilm Disc Attachment
|
N/A
|
Aug. 2001
|
|
Biofilm Disc Retrieval
|
N/A
|
Sept. 2001
|
|
Coral Assessment
|
Sept. 2001
|
Sept. 2001
|
|
Fish Census
|
Oct. 2001
|
Oct. 2001
|
|
Final Plate Attachment
|
N/A
|
Oct. 2001
|
|
5th
Quarterly Report
|
Oct. 2001
|
Oct. 2001
|
|
Coral Assessment
|
Dec. 2002
|
Jan. 2002
|
|
Fish Census
|
Jan. 2002
|
Jan. 2002
|
|
6th
Quarterly Report
|
Jan. 2002
|
Jan. 2002
|
|
Coral Assessment
|
Mar. 2002
|
Mar. 2002
|
|
Fish Census
|
Apr. 2002
|
Apr. 2002
|
|
Biofilm Labwork Complete
|
Apr. 2002
|
April 2002
|
|
7th
Quarterly Report
|
Apr. 2002
|
April 2002
|
|
Coral Assessment
|
June 2002
|
June 2002
|
|
Fish Census
|
July 2002
|
July 2002
|
|
Algal Attractant Plates Complete
|
July 2002
|
July 2002
|
|
8th Quarterly
Report
|
July 2002
|
July 2002
|
|
2nd Yearly Report
|
July 2002
|
July 2002
|
|
Algal Plate
Deployment
|
Aug. 2002
|
July 2002
|
|
Coral Assessment
|
Sept. 2002
|
Sept. 2002
|
|
Fish Census
|
Oct. 2002
|
Oct. 2002
|
|
9th
Quarterly Report
|
Oct. 2002
|
Oct. 2002
|
|
Coral Assessment
|
Dec. 2002
|
Rescheduled 2004
|
|
Fish Census
|
Jan. 2003
|
Jan. 2003
|
|
10th
Quarterly Report
|
Jan. 2003
|
Jan. 2003
|
|
Coral Assessment
|
Mar. 2003
|
March-June 2003
|
|
Fish Census
|
Apr. 2003
|
Apr. 2003
|
|
11th
Quarterly Report
|
Apr. 2003
|
Apr. 2003
|
|
Coral Assessment
|
June 2003
|
Rescheduled 2004
|
|
Algal Plate Retrieval
|
June. 2003
|
Rescheduled 2004
|
|
Fish Census
|
July 2003
|
July 2003
|
|
Contract Completion Report
|
July 2003
|
July 2003
|
|
|
Latitude Longitude Quad Label
26 03.369 80 05.798 1
26 03.342 80 05.797 2
26 03.324 80 05.798 3
26 03.305 80 05.804 4
26 03.285 80 05.804 5
26 03.261 80 05.800 6
26 03.241 80 05.804 7
26 03.226 80 05.799 8
26 03.205 80 05.798 9
26 03.190 80 05.799 10
26 03.375 80 05.770 11
26 03.360 80 05.779 12
26 03.288 80 05.744 13
26 03.273 80 05.782 14
26 03.170 80 05.801 15
26 03.177 80 05.784 16
26 03.161 80 05.777 17
26 03.142 80 05.759 18
26 03.143 80 05.778 19
26 03.161 80 05.756 20
26 03.183 80 05.760 21
26 03.202 80 05.764 22
26 03.219 80 05.748 23
26 03.226 80 05.756 24
26 03.250 80 05.754 25
26 03.267 80 05.762 26
26 03.272 80 05.744 27
26 03.286 80 05.758 28
26 03.312 80 05.758 29
26 03.326 80 05.760 30
26 03.344 80 05.767 31
26 03.357 80 05.750 32
26 03.190 80 05.777 33
26 03.207 80 05.779 34
26 03.224 80 05.780 35
26 03.242 80 05.777 36
26 03.257 80
05.782 37
26 03.291 80
05.782 38
26 03.320 80
05.780 40
26 03.307 80
05.778 39
26 03.373 80 05.749
Extra RBs (3 Reef Ball ‘quad’ w/ a broken RB)
26 03.382 80
05.746 Extra RBs
|
|
(note area of
USS Memphis grounding trench and DPEP damage control pins: CP1, 2, & 3).
(19 August –
18 October 2000)
The first quarter was a downtime for the project awaiting
deployment.
(19 October –
19 January 2000)
11/14-16/00
The contractors for Reefball
deployment arrived and loaded the balls on a barged moored at the Navy's dock.
Pat Quinn acted as a liaison between the contractors and NSU.
11/15/00
A meeting was held at NSUOC at
0900 hrs with OC and Broward County personnel to discuss the logistics of the
deployment scheduled for 11/17/00. In attendance were Ken Banks, Pamela
Fletcher, Joe Ligas, and Lou Fisher from Broward County and Richard Spieler,
Dave Gilliam, Pat Quinn, Elizabeth Glynn, Dan Fahy, Brian Walker, Paul Arena,
Lance Jordan, and Lance Robinson from OC.
11/17/00
The reef balls were deployed. The
Broward County boat Monitor was used to set buoys for the barge to use as marks
for setting quads (four reef balls). Above-mentioned OC personnel were on board
the Panacea. NCRI's boat Researcher was used to ferry news media to the site
and back. The first quad was deployed at approximately 0720 hrs and the last at
approximately 1410 hrs.
11/27/00
Pat Quinn and Elizabeth Glynn
dove on the site from R/V Researcher and started searching for the ends of the
quad lines, labeling the quads and mapping the grid. Ten quads were labeled. Lance Robinson and Dan Fahy crewed the
boat.
11/27/00 – 12/13/00
Multiple dive cancellations were
required due to weather.
12/14/00
Pat Quinn and Elizabeth Glynn
dove the site from the R/V Researcher to continue searching for the ends,
mapping and labeling. Twelve more quads were labeled. Lance Robinson and Judy
Robinson crewed the boat.
12/15/00
Richard Spieler and Ken Banks
decided, due to forecast weather and holiday scheduling, to postpone any
redeployment until the first of the year and that OCN personnel would
accomplish a full site map by early January.
01/04/01
Pat Quinn and Elizabeth Glynn
dove the deployment site trying to map the southern part of the "middle
row". One dive was made and the rest were cancelled due to high winds and
building seas. Brian Walker and Dan Fahy crewed Panacea.
01/06/01
Pat Quinn, Judy Robinson, and
Brian Walker on Panacea surveyed the deployment site visually finding Reefballs
from the surface and recording GPS coordinates.
01/07/01
Richard Spieler and Pat Quinn met
to discuss the apparent disarray of the grid.
1/08/01
Richard Spieler and Pat Quinn
discussed the arrangement of the grid with other members of the lab and
scheduled additional diving to confirm the grid and obtain more detailed
information on placement and distances.
01/11/01
Pat Quinn, Elizabeth Glynn, Dan
Fahy and Judy Robinson made four dives on the deployment site measuring
distances, retagging quads, and marking the quads for GPS coordinates.
Thirty-five quads were mapped. Brian Walker piloted Panacea.
01/12/01
Pat Quinn and Elizabeth Glynn
dove the deployment site to continue the work from 01/11/01 and finished
mapping the last 5 quads. Paul Arena and Dan Fahy crewed Panacea.
01/12/01
Richard Spieler and Pat Quinn met
to discuss the revised map of the grid.
01/15/01
Richard Spieler, Pat Quinn and
the rest of the members of the lab met to discuss the arrangement of the quads
and potential work schedule for moving the quads.
01/18/01
Meeting at DPEP.
Richard Spieler, Pat Quinn, and
Elizabeth Glynn met with Ken Banks, Pamela Fletcher, Joe Ligas, Lou Fisher, and
Dave Stout to discuss the number and logistics of moving quads.
(20 January
2001 – 27 April 2001)
01/24/01
Pat Quinn and Joe Ligas discussed proposed cable lengths and
techniques for moving the reef balls using balls located on the NSUOC property.
01/25/01
Richard Spieler, Richard Dodge, Andrew Rogerson, Pat Quinn,
Elizabeth Glynn and Judy Robinson met with Dr. Aileen Morse to discuss the CCA
extract and how it could be incorporated into the experimental design.
01/26/01
Pat Quinn and Elizabeth Glynn dove on the second reef near
the Memphis grounding site and found abundant colonies of Montastrea cavernosa that should be suitable for coring and control
monitoring. Dan Fahy piloted Panacea
and Rob Baron crewed.
Pat Quinn and Elizabeth Glynn started making concrete plugs
for the corals that will be cored.
01/29/01
Richard Spieler met with his lab personnel to discuss the
upcoming deployment.
01/30/01
Broward County personnel met at NSUOC for testing of
underwater communication masks to be used during the deployment. Joe Ligas gave
Pat Quinn a brief lesson.
01/31/01
Email communication between Richard Spieler, Ken Banks, Pat
Quinn and Elizabeth Glynn to set tentative dates to start moving the reef
balls. Cables should arrive 02/05/01 and could start moving reef balls
02/06/01.
02/01/01
Joe Ligas, Pat Quinn and Elizabeth Glynn discussed updated
procedures for moving the reef balls.
Pat Quinn supplied coordinates and labeling scheme to Ken Banks via
email.
02/05/01
Richard Spieler and Pat Quinn discussed the Memphis project
and projected work for the week with the other members of the lab.
Pat Quinn and Elizabeth Glynn finished making plugs for the
donor corals. They also made a ‘separator bar’ for moving of the reef balls.
02/06/01
Ken Banks communicated with Elizabeth Glynn via email that
he will schedule Thursday the 8th to start moving reef balls.
Richard Spieler and Elizabeth Glynn agreed to start coring
the donor corals and this information was passed along to Pat Quinn via phone.
02/12/01
Richard Spieler and Pat Quinn discussed the Memphis project
and projected work for the week with the other members of the lab.
02/14/01
Richard Spieler, Pat Quinn, Elizabeth Glynn and Ken Banks
discussed, via phone, starting to move the reef balls Sunday Feb. 18 weather
permitting. Also discussed was movement of the quads to ensure all of the
quads, direction and distance were considered.
02/19/01
Richard Spieler and Pat Quinn discussed the Memphis project
and projected work for the week with the other members of the lab.
02/21/01
Pat Quinn, Elizabeth Glynn, Dan Fahy, and Rob Baron worked
on positioning Reef Balls within quads using four 100 lb. lift bags. Five dives
were made using Panacea and 7 quads were positioned.
2/22/01
Pat Quinn, Elizabeth Glynn, Dan Fahy, and Rob Baron dove the
2nd reef north of Port Everglades looking for colonies of Diploria clivosa for coring. Two dives
were made using Panacea.
02/28/01
Pat Quinn, Elizabeth Glynn, Paul Arena, Rob Baron, Brian
Walker, Brian Buskirk, Dan Fahy and Fleur Harttung worked on positioning Reef
Balls within quads using five 100 lb. lift bags. Three dives were made using
Panacea and 8 quads were positioned.
03/03/01
Elizabeth Glynn and Lance Jordan assisted Ken Banks, Joe
Ligas and Lou Fisher in trying out the methods discussed in the meeting of
01/18/01. Two Reef Balls were moved and the trip was terminated due to building
seas.
03/09/01
Pat Quinn, and Elizabeth Glynn assisted Ken Banks, Joe
Ligas, Pam Fletcher, and Lou Fisher in moving 2.5 quads (10 Reef Balls) to the
correct locations. Two Reef Balls were moved at a time using two lift bags, two
cum-a-longs, one 2x4 spreader bar and various cables. Quinn, Glynn, Banks and
Ligas were divers while Fletcher and Fisher crewed the Monitor.
03/12/01
Richard Spieler, Richard Dodge, Pat Quinn, Elizabeth Glynn
and Dave Gilliam met at NSUOC to discuss the use of the boats, photography
equipment, methods, and personal schedules in relation to the Memphis project.
03/14/01
Pat Quinn, Elizabeth Glynn, Dr. Dave Gilliam, Susan Thornton
and Brian Ettinger started drilling coral transplants from the 2nd
reef (west of the Reef Ball deployment site). Glynn and Gilliam drilled,
Thornton and Ettinger photographed and plugged corals and Quinn crewed
Researcher with Capt. Lance Robinson. Eight cores were taken and placed in the
Reef Balls to be attached later. Eight dives were made.
03/16/01
Pat Quinn, Elizabeth Glynn, Paul Arena, Brian Ettinger and
Brian Walker epoxied the 8 coral transplants onto the Reef Balls. Two dives
were made using Panacea.
03/19/01
Richard Spieler and Pat Quinn discussed the Memphis project
and projected work for the week with the other members of the lab. Spieler and Quinn also tried various
techniques for creating small fill in the Reef Balls
03/23/01
Pat Quinn and Elizabeth Glynn assisted Ken Banks, Joe Ligas
and Lou Fisher in moving three quads to the correct locations. Two Reef Balls were
moved at a time using two lift bags, two come-a-longs, one 2x4 spreader bars
and various cables. Quinn, Banks and Ligas were divers while Glynn and Fisher
crewed the Monitor. Five dives were made.
04/01/01
Pat Quinn, Elizabeth Glynn, and Dan Fahy assisted Ken Banks
and Joe Ligas in moving three quads to the correct locations. An entire quad
was moved at a time using three lift bags, four come-a-longs, three 2x4
spreader bars and various cables. Quinn, Fahy and Banks were divers while Glynn
and Ligas crewed the Monitor. Three dives were made.
04/02/01
Richard Spieler and Pat Quinn discussed the Memphis project
and projected work for the week with the other members of the lab.
Elizabeth Glynn, Dan Fahy, Brian Buskirk, Fleur Harttung and
Ryan Moyer dove the 2nd reef mapping donor corals for coring. Three
dives were made using Panacea.
04/04/01
Elizabeth Glynn, Dan Fahy and Rob Baron surveyed the reef
near the Memphis grounding looking for donor corals to core. One dive was made
by Glynn and Baron using OSC’s vessel Lucy Forman. Fahy crewed the Lucy.
04/09/01
Richard Spieler and Pat Quinn discussed the Memphis project
and projected work for the week with the other members of the lab.
04/10/01
Pat Quinn, Elizabeth Glynn and Dave Gilliam assisted Ken Banks
and Joe Ligas in moving one quad to the correct location. Site location problems prevented more quads
from being moved. Quinn, Gilliam and
Ligas were divers while Banks and Glynn crewed the Monitor. Multiple dives were
made.
04/16/01
Richard Spieler and Pat Quinn discussed the Memphis project
and projected work for the week with the other members of the lab.
04/17/01
Pat Quinn, and Elizabeth Glynn assisted Ken Banks, Joe Ligas
and Dave Stout in moving four quads to the correct locations. Quinn, Glynn,
Banks, and Stout were divers while Ligas crewed the Monitor. Four dives were
made.
04/23/01
Richard Spieler, Pat Quinn and Elizabeth Glynn discussed the
Memphis project and projected work for the week with the other members of the
lab. Spieler, Quinn and Glynn met again
to discuss various techniques for attaching settlement plates and attractants
to the reef balls. Method trials will
be made for plate attachment using other Reef Balls from previous projects.
04/25/01
Pat Quinn, Elizabeth Glynn, Dave Gilliam, Dan Fahy, Jamie
Vernacchio, Brian Ettinger and Rob Baron dove the 2nd reef near the
Memphis grounding site mapping and
tagging corals for coring or controls. Capt. Lance Robinson and Susan Thornton
crewed the Researcher. Four dives were made.
04/27/01
Richard Spieler and Pat Quinn made a cone using ¾” plastic
cage material to function as small fill in a Reef Ball. Spieler authorized the purchase of cage
material for 60 cones.
(01 May 2001 –
19 July 2001)
05/01/01
Dr. Richard Spieler authorized purchase of 220 blocks for
large fill and 65 ft. of ¾” plastic cage material for small fill in the RBs.
Dr. Richard Spieler and Pat Quinn discussed various types of
iron product to be added to the settlement plates as an attractant. Dr. Spieler
ordered two different types of iron products for testing.
05/04/01
Dr. Richard Spieler and Pat Quinn received the block and
cage material.
05/07/01
Dr. Richard Spieler met with his lab personnel to coordinate
the making of small fill using the cage material cut into cones. The cones were
completed that week.
Dr. Richard Spieler, Pat Quinn and Elizabeth Glynn met to
discuss the logistics of adding fill, drilling coral cores, and attaching
settlement plates.
05/09/01
Dr. Richard Spieler, Pat Quinn and personnel from Industrial
Divers discussed various mixtures of concrete that could be used to attach the
settlement plates to the RBs while underwater.
05/11//01
Dr. Richard Spieler and Pat Quinn coated a settlement plate
with cement and embedded calcium carbonate and iron. The plate was submerged in
the marine environment as a methods test.
05/23/01
Pat Quinn and Rob Baron dove the Memphis RBs to practice
attaching settlement plates and small fill. Sixteen block for large fill was
dropped near a quad. One dive was made and the remainder of the work was
cancelled due to thunderstorms. Paul Arena captained Panacea and Elizabeth
Glynn crewed.
05/25/01
Pat Quinn, Elizabeth Glynn, Dan Fahy and Brian Buskirk
dropped 32 blocks for large fill in 2 quads. Five dives were then made at the
grounding site to map corals for donors to be drilled and controls from
Panacea.
05/27/01
Elizabeth Glynn and Dan Fahy dove the grounding site to map
donor corals from drilling. Two dives were made from the Lucy Forman.
05/30/01
Pat Quinn, Elizabeth Glynn, Dan Fahy and Brian Buskirk
dropped 64 blocks for large fill in 4 quads. Quinn and Buskirk filled two quads
with 16 blocks each. Elizabeth Glynn and Dan Fahy then dove the grounding site
to map and tag donor corals. Four dives were made from Panacea.
05/31/01
Pat Quinn, Elizabeth Glynn, Dan Fahy and Brian Buskirk
dropped blocks near the quads for large fill. Quinn and Buskirk filled four
quads with 16 blocks each and measured the distance between specific quads.
Elizabeth Glynn and Dan Fahy then dove the grounding site to
map and tag donor corals.
Five dives were made from Panacea.
06/01/01
Pat Quinn, Elizabeth Glynn, Ken Banks, Joe Ligas and Lance
Jordan moved RBs using Monitor. Quinn, Banks and Jordan made three dives moving
quads 5 and 27. Ligas captained Monitor and Glynn crewed.
06/03/01
Elizabeth Glynn, Dave Gilliam, and Susan Thornton dove the
grounding site to photograph donor and control corals. Two dives were made from
Researcher. Lance Robinson captained.
06/04/01
Dr. Richard Spieler and Pat Quinn discussed the Memphis
project and projected work for the week with the other members of the lab.
Pat Quinn, Elizabeth Glynn, Dave Gilliam and Brian Ettinger
dove the grounding site on the Lucy Forman to drill the donor corals. The drill
did was not working properly and the day was aborted to troubleshoot the
problem.
06/05/01
Pat Quinn, Ken Banks, Joe Ligas and Amy Hall moved RBs using
Monitor. Quad 1 was moved and quads 5, 27 and 36 had individual balls
repositioned. Four dives were made.
Elizabeth Glynn, Susan Thornton, Dave Gilliam and Brian
Ettinger dove the grounding site to drill donor corals. Three dives were made
from the Lucy Forman. The drill was not
working properly and the day was aborted.
A replacement drill was obtained the next day.
06/06/01
Pat Quinn, Paul Arena, and Rob Baron made two trips on
Panacea to drop 72 blocks for large fill on 12 quads.
06/08/01
Pat Quinn, Elizabeth Glynn, Dan Fahy and Brian Ettinger dove
the grounding site to drill corals and place the cores in RBs. Five dives were
made from the Lucy Forman.
06/11/01
Dr. Richard Spieler and Pat Quinn discussed the Memphis
project and projected work for the week with the other members of the lab.
Elizabeth Glynn, Dave Gilliam, Susan Thornton and Brian
Ettinger dove the grounding site to finish photographing donor corals prior to
drilling. Three dives were made from Researcher. Lance Robinson captained.
06/12/01
Pat Quinn, Elizabeth Glynn, Brian Ettinger and Kevin Helmle
dove the grounding site to drill corals and place the cores in RBs. Four dives were made from the Lucy Forman.
06/13/01
Pat Quinn, Elizabeth Glynn and Dan Fahy dove the grounding
site to drill donor corals and place the cores in RBs. Quinn and Glynn made
three dives were made from the Lucy Forman and Fahy captained.
06/15/01
Pat Quinn, Elizabeth Glynn, Dave Gilliam, Brian Ettinger and
Kevin Helmle dove the grounding site to drill donor corals and place the cores
in the RBs. Five dives were made from the Lucy Forman. Due to lack of air and
time, 6 cores were left inside a RB of quad 23 to be moved later. This
completed drilling of corals to be used as attactants.
06/16/01
Elizabeth Glynn, Dave Gilliam and Brian Ettinger dove quad
23 to recover the cores left from the previous day. These cores were placed in
their correct quads. This completed the distribution of corals to the RBs to be
used as attractants. The corals will still need to be permanently attached and
photographed. Glynn and Gilliam made one dive from the Lucy Forman and Ettinger
captained.
06/18/01
Dr. Richard Spieler and Pat Quinn discussed the Memphis
project and projected work for the week with the other members of the lab.
06/19/01
Pat Quinn, Elizabeth Glynn, Dan Fahy and Mike Hoke dove the
RBs to epoxy cores into the RBs and photograph. Four dives were made from the
Lucy Forman.
06/20/01
Pat Quinn, Elizabeth Glynn, Judy Robinson, and Ryan Moyer
dove the RBs to epoxy cores into the RBs, photograph the cores and place small
fill in quads 5 and 14. Four dives were made from the Lucy Forman.
06/21/01
Pat Quinn, Elizabeth Glynn, Amy Hall and Ryan Moyer dove the
RBs. Quinn and Hall made two dives to add fill to quads 6, 8 and 36. Glynn and
Moyer made two dives to epoxy cores into the RBs and take photographs. Four dives were made from Panacea
06/22/01
Elizabeth Glynn, Dan Fahy, Brian Ettinger and Jamie
Vernacchio dove the RBs to epoxy and photograph the cores in quads 9, 10, 15,
17, 23 and 24. Two dives were made from
Panacea and additional work was aborted due to thunderstorms.
06/24/01
Elizabeth Glynn, Dan Fahy, Ryan Moyer and Heather Halter
dove the RBs to epoxy and photograph the remaining cores. This completed the
addition of corals to the RBs as attractants.
The personnel then moved to the grounding site to epoxy
plugs into the donor corals and take photographs.
A total of four dives were made from the Lucy Forman.
06/25/01
Dr. Richard Spieler and Pat Quinn discussed the Memphis
project and projected work for the week with the other members of the lab.
Pat Quinn, Elizabeth Glynn, Dan Fahy, Rob Baron, Fleur
Hartung, and Amy Hall dove the Memphis RBs adding small and large fill to
quads. Four dives were made from Panacea and additional work was aborted due to
thunderstorms.
06/26/01
Dr. Richard Spieler, Pat Quinn and personnel from the Rinker
Materials Corp. discussed the cement mix used to make the RBs as this same mix
will be used to attach the coral attractants to the settlement plates.
Elizabeth Glynn, Dan Fahy, and Amy Hall dove the 2nd
reef near the Memphis grounding site to epoxy plugs into the donor corals and
photograph donors and controls. Two dives were made at this location.
Pat Quinn and Rob Baron dove the Memphis RBs adding small
and large fill to the quads. One dive was made at this location.
Panacea was used the entire day.
07/02/01
Dr. Richard Spieler and Pat Quinn coated a settlement plate
with a cement mixture covered with calcium carbonate. When dry, the plate was
placed in the marine environment as a methods test.
07/06/01
Pat Quinn, Elizabeth Glynn, Paul Arena and Fleur Hartung
dove the RBs to add small and large fill to the quads. This completed filling
the quads for the complexity aspect. The personnel then moved to the grounding
site to epoxy plugs into the donor corals and take photographs. Five dives were
made from Panacea.
07/09/01
Pat Quinn and Judy Robinson discussed the substrate coatings
to be used on the biofouling plates. It
was determined to follow the same coating methods as used on the larger
settlement plates. Dr. Andrew Rogerson agreed with this procedure.
07/19/01
Judy Robinson and NSUOC personnel constructed the biofouling
plate arrays with just concrete, concrete and quarry rock, concrete and iron,
and glass slides. Seventy-two biofouling plates with the appropriate substrate
types and 24 glass slides make up the four arrays.
(20 July 2001
– 11 October 2001)
07/29/01
Richard Spieler and Pat Quinn discussed personnel
assignments and logistics of deploying the 320 settlement plates on the quads.
It was decided to put all 320 plates in the water before attaching the plates
to the Reef Balls.
07/30/01
Pat Quinn, Brian Walker, Judy Robinson and Lance Robinson
placed 96 settlement plates on Quads 1-10, 15 and 33. The plates were stood
upright on the substrate and against the Reef Balls to which they will be later
cemented. J. Robinson lowered the plates to Quinn and Walker who made 2
dives. L. Robinson captained
Researcher.
After the plates were deployed, Richard Spieler and Pat
Quinn met at NSUOC to discuss personnel and logistics for the following day.
07/31/01
Pat Quinn, Brian Walker, Judy Robinson, Lance Robinson and
Amy Hall placed 104 settlement plates on Quads 16-27 and Q 13. Walker and J.
Robinson lowered the plates to Quinn and Hall during one dive. Plates for the
13 quads were deployed on one dive. Twenty-four plates remained on board for
three more quads so it was decided to return to NSUOC and load the remaining 96
plates. At the dock, Paul Arena replaced Amy Hall.
Arena and J. Robinson lowered the plates to Quinn and Walker
on two dives that completed the plate deployment. L. Robinson captained Researcher.
Andrew Rogerson and Judy Robinson met to discuss the
deployment site of biofilm discs and a collection schedule.
08/06/01
Pat Quinn, Elizabeth Glynn, and Amy Hall started cementing
settlement plates onto the Reef Balls. Two dives were made from the Lucy
Forman. The first dive was on Quad 1
and the second dive on Quad 12.
08/08/01
Pat Quinn, Elizabeth Glynn and Brian Walker cemented
settlement plates onto Reef Balls. Two dives were made from the Lucy Forman.
The first dive was on Quad 31 and the second dive on Quad 11.
08/09/01
Pat Quinn, Elizabeth Glynn and Amy Hall cemented settlement
plates onto Reef Balls. Two dives were made from the Lucy Forman. The first
dive was on Quad 32 and the second dive on Quad 30.
08/10/01
Richard Spieler and Pat Quinn discussed the upcoming coral
spawning and decided to have divers orient the plates in the correct position
for settlement per the experimental design then, starting with the top plates,
cement them onto the Reef Balls.
08/11/01
Pat Quinn, Elizabeth Glynn, Dan Fahy and Brian Buskirk
positioned the remaining settlement plates on the Reef Balls in preparation of
the upcoming spawning. Plates were laid
flat on top of the Reef Balls and placed upright against the sides. In
addition, top plates near the coral transplants were cemented on Quads 7-10,
14, 15, 28, 29 and 37. Three dives were made from Panacea.
08/12/01
Pat Quinn, Dave Gilliam, Brian Walker and Rob Baron cemented
top plates onto Reef Balls. Four dives were made from Panacea attaching plates
to Quads 2-10, 28, 29 and 39.
08/13/01
Richard Spieler and Pat Quinn met with other lab personnel
to discuss actual procedures of mixing cement underwater and application of the
settlement plates onto the Reef Balls.
Andrew Rogerson and Judy Robinson met to discuss methodology
for preparation of biofilm discs to be used in counting. It was agreed to use
previous designed methods from the preliminary study.
08/14/01
Pat Quinn, Robin Sherman, Dan Fahy and Amy Hall cemented top
plates onto Reef Balls. Four dives were made from Panacea attaching plates to
Quads 13, 15, 17, 19 and 25-27. Paul
Arena captained Panacea and Rob Baron lowered bags of cement to divers as
needed.
Judy Robinson and Lance Robinson attached four arrays of 24
discs coated with different attractants to the Reefballs. One dive was made
from the Researcher with Brian Ettinger as captain.
08/15/01
Richard Spieler, Pat Quinn, Dan Fahy, Amy Hall and Brian
Buskirk cemented plates onto Reef Balls. Four dives were made during which the
remaining top plates were attached and work began on cementing side
plates. Paul Arena captained Panacea
and Rob Baron lowered bags of cement to divers as needed.
Judy Robinson and Lance Robinson collected 12 discs from the
Reef Balls. One dive was made from the Researcher with Brian Ettinger as
captain.
08/17/01
Judy Robinson and Lance Robinson collected 12 discs from the
Reef Balls. One dive was made from the Researcher with Brian Ettinger as
captain.
08/19/01
Pat Quinn, Dave Gilliam, Dan Fahy and Brian Buskirk cemented
plates onto Reef Balls. Four dives were made attaching plates to Quads 2-7, 14,
34, 35 and 37-40. Richard Spieler captained Panacea and Rob Baron lowered bags
of cement to divers as needed.
08/20/01
Pat Quinn, Elizabeth Glynn, Dan Fahy, Amy Hall and Brian
Buskirk cemented plates onto Reef Balls. Five dives were made attaching plates
to Quads 8-10, 13, 15, 16, 18, 21-28, 31 and 33. Richard Spieler captained
Panacea and Paul Arena lowered bags of cement to divers as needed.
08/24/01
Judy Robinson and Lance Robinson collected 12 discs from the
Reef Balls. One dive was made from the Researcher with Brian Ettinger as
captain.
08/28/01
Pat Quinn, Dan Fahy and Fleur Harttung surveyed the quads to
ensure all plates were still attached and had been correctly placed. Of the 320 settlement plates attached to the
Reef Balls, only 2 had come loose, one each on Quads 6 and 32. Both were side
plates. Two dives were made from Panacea and Elizabeth Glynn crewed.
08/30/01
Judy Robinson and Lance Robinson collected 12 discs from the
Reef Balls. One dive was made from the Researcher with Brian Ettinger as
captain.
09/04/01
Dave Gilliam and Lance Robinson collected 12 discs from the
Reef Balls. One dive was made from the Researcher with Brian Ettinger as
captain.
09/12/01
Richard Spieler and Pat Quinn discussed preliminary
methodology for conducting the fish community surveys.
09/20/01
Elizabeth Glynn, Dan Fahy and Susan Thornton photographed
donor and control corals at the Memphis grounding site. One dive was made from
Researcher with Brian Ettinger as captain and Dave Gilliam as crew.
09/25/01
Elizabeth Glynn, Dan Fahy and Fleur Harttung photographed
donor and control corals at the Memphis grounding site. Two dives were made
from Panacea with Pat Quinn as captain.
09/28/01
Elizabeth Glynn and Dan Fahy photographed donor and control
corals at the Memphis grounding site. Two dives were made from the Lucy Forman
with Pat Quinn as captain.
10/02/01
Elizabeth Glynn, Pat Quinn, Fleur Harttung and Brian Buskirk
photographed transplant corals on the Reef Balls. Three dives were made from Panacea.
10/03/01
Elizabeth Glynn and Amy Hall photographed transplant corals
on the Reef Balls. Two dives were made from the Lucy Forman with Pat Quinn as
captain.
10/05/01
Richard Spieler and Pat Quinn met with lab personnel to
discuss methodology and dive schedule of the fish monitoring.
10/06/01
Richard Spieler and Pat Quinn discussed final details of
methodology and personnel for the fish monitoring.
10/07/01
Pat Quinn, Elizabeth Glynn, Dan Fahy and Robin Sherman
conducted the first fish community monitoring on the quads. Four dives were
made from Panacea and data were collected from Quads11-13, 16-34.
(15 October
2001 – 11 January 2002)
10/12/01
Richard Spieler and Pat Quinn met with lab personnel to review
methodology and dive scheduling for the fish monitoring.
10/15/01
Pat Quinn, Elizabeth Glynn, Dan Fahy and Brian Buskirk
finished the first fish community monitoring on the quads. Five dives were made
from Panacea and data were collected from Quads1-10, 14, 15, 35-40.
Additionally, photographs were taken of the coral transplants on Quads 5-8.
10/16/01
Pat Quinn and Rob Baron finished cementing the settlement
plates onto the Reef Balls of Quads 6 and 32. One dive was made from Panacea
with D. Fahy as capt. and E. Glynn as crew.
12/05/01
Richard Spieler, Elizabeth Glynn and Pat Quinn met with lab
personnel to review methodology and dive scheduling for the corol monitoring.
12/12/01
Elizabeth Glynn and Heather Halter photographed donor and
control corals at the Memphis grounding site (CP2). Two dives were made from
Panacea with P. Quinn as capt. and D. Fahy as crew.
12/14/01
Elizabeth Glynn, Brian Buskirk and Fleur Harttung
photographed coral transplants on Quads 2-10, 14-17, 19, 33-40. Two dives were made
from Panacea with P. Quinn as capt.
Additionally, a thermograph was changed on Quad 36.
12/17/01
Elizabeth Glynn and Dan Fahy finished photographing the
coral transplants on Quads 1, 11-13, 18, 20-32. Two dives were made from
Panacea with P. Quinn as capt. and A. Hall as crew.
01/07/02
Richard Spieler and Pat Quinn met with lab personnel to
review methodology and dive scheduling for the second fish monitoring.
01/09/02
Richard Spieler, Pat Quinn and Lance Robinson finalized the
schedule for conducting the fish monitoring on the following day.
01/10/02
Richard Spieler, Pat Quinn, Elizabeth Glynn, Dan Fahy, Brian
Buskirk, Amy Hall, Fleur Harttung and Rob Baron conducted the second fish
community monitoring on the quads. Seven dives were made from Researcher and
data were collected from Quads 1-20 and 22-40. Lance Robinson captained
Researcher.
01/11/02
Elizabeth Glynn and Dan Fahy finished photographing the
donor and control corals at the Memphis grounding site. Pat Quinn and Fleur
Harttung completed the second fish community monitoring and data were collected
from Quad 21. Four dives were made from Panacea.
(11 January –
April 11, 2002)
01/18/02
Richard Spieler and Pat Quinn discussed conducting a maintenance
dive on the Reef Balls to reattach plates and quad labels.
01/22/02
Richard Spieler, Pat Quinn, Dan Fahy and Rob Baron
reattached settlement plates on Quads 3, 7, 16, 30, 32, 33 and 38. Three dives
were made from Panacea.
01/25/02
Elizabeth Glynn and Dan Fahy dove the Memphis grounding site
to complete control coral photographs. One dive was made from Panacea with F.
Harttung as Captain.
02/26/02
Pat Quinn and Elizabeth Glynn changed a thermograph on Quad
36 and examined Quad 32 for loose settlement plates. One dive was made from
Panacea with Fleur Harttung as Captain and Dan Fahy as crew.
03/11/02
Richard Spieler, Elizabeth Glynn and Pat Quinn met with lab
personnel to discuss scheduling for photographing the transplant, donor and
control corals.
03/13/02
Elizabeth Glynn, Dan Fahy, Amy Hall and Fleur Harttung
photographed transplant corals on the Reef Balls. Four dives were made from
Panacea.
03/15/02
Elizabeth Glynn, Ryan Moyer and Brian Buskirk photographed
donor and control corals at the Memphis grounding site (CP2). Two dives were made from Panacea with P.
Quinn as Captain. Additional work was aborted to due injury, weather conditions
and equipment problems.
03/16/02
Elizabeth Glynn and Dan Fahy photographed donor and control
corals at the Memphis grounding site (trench). Two dives were made from the R/V
Lucy Forman.
03/26/02
Elizabeth Glynn and Dan Fahy dove the Memphis grounding site
(CP2) to photograph corals and record GPS coordinates for large donor and
control corals. One dive was made from Panacea with Paul Arena as Captain and
Rob Baron as crew.
03/28-03/29/02
Judy Robinson scraped, filtered and made slides of the
accumulated substance on the remaining collected biofilm plates (4).
03/29/02
Elizabeth Glynn and Heather Halter dove the Memphis
grounding site (CP1) to finish photographing corals and record GPS coordinates.
One dive was made from Panacea with F. Harttung as Captain.
04/01/02
Richard Spieler and Pat Quinn met with lab personnel to
review methodology and dive scheduling for the fish monitoring. Scheduled date
to conduct the monitoring is 04/08/02.
04/02/02
Andrew Rogerson and Judy Robinson discussed the methods in
which the slides will be counted. Four
slides were examined and the bacterial cells counted.
04/07/02
Richard Spieler and Pat Quinn canceled the scheduled
04/08/02 monitoring due to weather. Pat Quinn contacted lab personnel and
rescheduled for 04/10/02.
04/09/02
Richard Spieler and Pat Quinn canceled the scheduled
04/11/02 monitoring due to weather. Next scheduled date is TBD, weather
contingent.
(April 2002 -
July 2002)
04/19/02
Richard Spieler and Pat Quinn met with lab personnel to
discuss scheduling and procedures for the third fish community monitoring.
04/22/02
Pat Quinn, Elizabeth Glynn, Dan Fahy, Brian Buskirk, Rob
Baron and Paul Arena conducted the third fish community monitoring on the
quads. Six dives were made from the Panacea and data were collected from all 40
quads. Tags with the appropriate quad number were replaced on each quad.
05/02/02
Pat Quinn and Ryan Moyer dove on Quad 15 looking for coral
recruits. Another dive was made on Quad 32 to reattach a settlement plate. The
plate was found, and put in place, but
was not attached due to time constraints returning to the port before Fleet
Week closure. Two dives total were made from the Researcher with B. Ettinger as
Captain and D. Gilliam as crew.
06/04/02
Pat Quinn and Dan Fahy reattached settlement plates on Quads
32 and 39. One dive was made from the Panacea with F. Harttung as Captain and
R. Baron as crew.
06/13/02
Richard Spieler and Pat Quinn discussed potential designs
for settlement plates to be used in the Crustose Coralline Algae (CCA) study.
06/17/02
Andrew Rogerson and Judy Robinson discussed the problem with
cover slip detachment on some of the iron and CaCo2 slides. Judy
Robinson continued bacterial counts.
06/18-26/02
Pat Quinn worked on several potential design structures for
settlement plates to be used in the CCA study.
06/19/02
Andrew Rogerson and Judy Robinson developed methodology to
prevent cover slip detachment of the iron and CaCo2 slides. Judy
Robinson continued bacterial counts.
06/20/02
Judy Robinson completed bacterial counts on samples with no
treatment. Remaining bacterial counts continue.
06/24/02
Judy Robinson completed bacterial counts on samples on glass
slides. Remaining bacterial counts continue.
06/27/02
Judy Robinson completed bacterial counts on samples with
CaCo2 treatment. Remaining bacterial counts continue.
07/1/02
Andrew Rogerson and Judy Robinson discussed the bacterial
density calculations. Judy Robinson
completed bacterial counts with iron treatment.
07/2/02
Andrew Rogerson and Judy Robinson discussed the analysis and
write-up for final report.
Richard Spieler and Pat Quinn discussed finalized structure
for settlement plates to be used in CCA study.
07/03/02
Elizabeth Glynn, Pat Quinn, Dan Fahy and Ryan Moyer
photographed transplant corals on Quads 1-19 and 25-40. Three dives were made
from the Panacea. The remaining dives
were cancelled due to weather.
07/05/02
Elizabeth Glynn, Pat Quinn, photographed the tranplant
corals on Quads 20-24. One dive was made from the Panacea with D. Fahy as
Captain and R. Moyer as crew. The remaining dives were cancelled due to
equipment failure.
07/05-15/02
Pat Quinn made 32 settlement plate modules to be used in the
CCA study.
07/07/02
Elizabeth Glynn, Pat Quinn and Dan Fahy photographed donor
and control corals at the Memphis grounding site. Three dives were made from
the Thompson. An additional dive was made by Pat Quinn and Dan Fahy to
photograph a coral recuit located on Quad 15.
07/10/02
Elizabeth Glynn, Pat Quinn and Dan Fahy finished
photographing donor and control corals at the Memphis grounding site. Three
dives were made from the Thompson.
07/17/02
Richard Spieler and Pat Quinn discussed potential deployment
sites for the CCA settlement plate modules and logistics for the actual
deployment.
07/18/02
Richard Spieler and Pat Quinn surveyed two potential study
areas for deployment of the CCA modules. Eight modules were placed in the Site
1 Control area and 8 were placed in the Site 1 Study area. Two dives were made
from Panacea with F. Harttung as Captain and P. Arena and D. Fahy as crew.
07/19/02
Richard Spieler, Pat Quinn and Robin Sherman dove Site 2
Control and Study areas to place sixteen CCA modules. Spieler, Quinn, Sherman
and Dan Fahy then dove Site 1 Control to remove any rubble around the CCA
control modules. Two dives were made from Panacea with F. Harttung as Captain
and P. Arena as crew.
07/21/02
Richard Spieler and Pat Quinn discussed the logistics for
conducting the fourth fish community monitoring on the quads.
07/22/02
Richard Spieler, Pat Quinn, Robin Sherman, Fleur Harttung,
Dan Fahy, Paul Arena, Brian Buskirk and Kristy Foster conducted the fourth fish
community monitoring on the quads. Six dives were made from the Panacea and
data were collected from all 40 quads.
(July 2002 - Oct 2002)
09/15/02
Elizabeth Glynn and
Dan Fahy dove the Memphis grounding site photographing donor and control
corals. Two dives were made from the Lucy Forman.
09/16/02
Elizabeth Glynn and
Dan Fahy dove the Memphis grounding site photographing donor and control
corals. One dive was made from Researcher with Lance Robinson as Captain and
Dave Gilliam as crew.
09/17/02
Elizabeth Glynn and
Dan Fahy dove the Memphis grounding site photographing donor and control
corals. Two dives were made from the Lucy Forman.
09/20/02
Pat Quinn and Lance
Robinson discussed the construction of a photographic framer to be used in
coral settlement assessment.
09/23/02
Lance Robinson
delivered the framer.
09/21/02
Richard Spieler and
Pat Quinn dicussed additional photography equipment needed for the coral
settlement aspect of the Memphis project. Richard Spieler ordered the
equipment.
09/28/02
Elizabeth Glynn, Dan
Fahy, Paul Arena and Fleur Harttung dove the Memphis Reef Balls photographing
transplant corals. Three dives were made from Panacea.
10/1/02
Richard Spieler and
Pat Quinn met with lab personnel to discuss scheduling and procedures for the
fourth fish community monitoring.
10/04/02
Pat Quinn and Lance
Robinson discussed modification of the framer to accept the additional
photographic equipment.
10/07/02
Lance Robinson
completed the needed modifications to the framer.
10/10/02
Pat Quinn cancelled
the scheduled fish community monitoring due to personnel shortage and equipment
failure.
10/15/02
Richard Spieler and
Pat Quinn scheduled the fish community monitoring to take place on 10/15/02,
weather permitting.
10/16/02
Richard Spieler and
Pat Quinn cancelled the scheduled fish community monitoring due to a Small
Craft Advisory. Personnel will be scheduled
daily to complete the monitoring when the advisory is cancelled.
10/22/02
Pat Quinn, Paul
Arena, Brian Buskirk and Arlo Hemphill conducted the fourth fish community
monitoring on the quads. Four dives were made from Panacea and data were
collected from all 40 quads.
(Nov 2002 –
Jan 2003)
12/00/03
Due to personnel
changes/availability and weather, coral transplant assessments were not
completed. Photographs will be taken as
soon as possible.
01/06/03
Richard Spieler and
Pat Quinn met with lab personnel to discuss scheduling and procedures for the
fifth fish community monitoring.
01/08/03
Pat Quinn, Paul
Arena, Brian Buskirk, Arlo Hemphill, and Fleur Harttung conducted the fifth
fish community monitoring on the quads. Five dives were made from Panacea and
data were collected from all 40 quads.
01/15/03
Richard Spieler and
Pat Quinn met to discuss techniques and scheduling for photographing of coral
settlement plates.
01/16/03
Richard Spieler, Pat
Quinn and Arlo Hemphill dove CCA Sites 1 and 2 to monitor the sites, place tags
on the reefs and take photographs of the settlement plates. Photographs were
completed on Site 1 and the Control reefs of Site 2, however, wave surge
prevented photographs from being taken on the Control reefs of Site 2. Two
dives were made from Panacea.
01/21/03
Pat Quinn, Amy Hall
and Arlo Hemphill dove the Memphis Reef Balls to photograph settlement plates.
Photographs were taken on Quads 2-10, 15, 17-19. Two dives were made from Panacea with F. Harttung as Captain.
01/30/03
Richard Spieler and
Pat Quinn dove North Site to photograph CCA plates at the Control and Study
areas. One dive was made at this site.
Richard Spieler and
Pat Quinn then made a dive on the Reef Balls to photograph settlement plates.
Photographs were taken on Quads 16 and 33-36. One dive was made with Arlo
Hemphill as Captain on Panacea for both dives.
(Feb 2003 –
Apr 2003)
02/04/03
Pat Quinn, Paul
Arena and David Bryan dove the Memphis Reef Balls to photograph settlement
plates. Photographs were taken on Quads
1-3, 11, 12, 14, 29, 30, 31, 36, 37, 39, 40.
Two dives were made from Panacea with F. Harttung as Captain.
02/05/03
Pat Quinn, Brian
Buskirk, and David Bryan dove the Memphis Reef Balls to photograph settlement
plates. Photographs were taken on Quads 13, 20-28, 32, 38. Three dives were made from Panacea with F.
Harttung as Captain.
02/26/03
Pat Quinn, David
Bryan and Arlo Hemphill dove the Memphis Reef Balls to photograph settlement
plates. Photographs were taken on Quads 4-10, 15-19, 33-35. Three dives were made from Panacea with P.
Arena as Captain.
03/03/03
Richard Spieler and
Pat Quinn met with lab personnel to discuss the logistics of photographing the
coral transplants.
03/05/03
Pat Quinn and David
Bryan dove the Memphis Reef Balls to photograph coral transplants. Photographs
were taken on Quads 1-10, 14-16, 33-39. Two dives were made from Panacea with
P. Arena as Captain.
03/06/03
Transplant, donor,
and control photographs were developed and reviewed. Results showed problems with equipment or techniques.
03/07/03
Richard Spieler, Pat
Quinn, Dave Gilliam and Elizabeth Glynn met to discuss equipment problems
experienced during the current transplant photography and techniques to correct
the problems.
03/06/03
Pat Quinn, Fleur
Harttung, and Brian Buskirk dove the Memphis Reef Balls to photograph
settlement plates. Photographs were taken on Quads 11-13, 18-32. Two dives were
made from Panacea with Arlo Hemphill as boat hand.
03/14/03
Pat Quinn, David
Bryan and Paul Arena dove the Memphis Reef Balls to retake photographs on the
settlement plates and transplants due to exposure and equipment problems on
previous dives resulting in unusable data. Settlement plate photographs were
taken on Quads 16, 18, 33-36 and transplant photographs were taken on Quads
1-10, 12, 15. Three dives were made
from Panacea with Meaghan Darcy as boat hand.
03/17/03
Richard Spieler, Pat
Quinn, Dave Gilliam and Elizabeth Glynn met again to discuss equipment problems
experienced during the transplant photography and other techniques to try and
correct the problems.
04/14/03
Richard Spieler and
Pat Quinn met with lab personnel to discuss the logistics for conducting the sixth
fish community monitoring.
Richard Spieler and
Pat Quinn discussed coral recruitment and statistical analysis and decided an
additional 12 months of research was required to adequately address the
research guidelines.
04/17/03
Pat Quinn, Brian
Buskirk, David Bryan and Jeremy Barnes conducted the sixth fish community
monitoring on the quads. Four dives were made from Panacea and data were
collected from all 40 quads. P. Arena
was Captain and F. Harttung was boat hand.
04/23/03
Pat Quinn, David
Bryan and Brian Buskirk dove the Memphis Reef Balls to photograph coral
transplants. Photographs were taken on Quads 1-17, 21, 23-40. Three dives were made from the R/V Thompson.
04/23/03
Pat Quinn and Dave
Gilliam met to review camera equipment used for the coral transplants.
Equipment changes were made in an attempt to correct continuing equipment
problems.
04/24/03
Pat Quinn, David
Bryan and Elizabeth Glynn dove the Memphis grounding site to photograph donor
and control corals. Photographs were taken of donor coral transplant plugs and
small controls. Three dives were made
from the R/V Thompson.
04/25/03
Transplant, donor,
and control photographs were developed and reviewed. Results show the current
camera equipment problems have been resolved. Completion of the coral
photographs and growth assessment will be as soon as possible.
Empty Small
Mean 12.71428571 Mean 14.42857143
Standard Error 0.362383904 Standard
Error 0.382630878
Median 13 Median 14
Mode 10 Mode 14
Standard Deviation 3.031921269 Standard
Deviation 3.201319604
Sample Variance 9.192546584 Sample
Variance 10.2484472
Kurtosis -0.247150572 Kurtosis -0.088612297
Skewness 0.229801765 Skewness 0.233415332
Range 14 Range 15
Minimum 6 Minimum 7
Maximum 20 Maximum 22
Sum 890 Sum 1010
Count 70 Count 70
Mixed Large
Mean 16.01428571 Mean 16.82857143
Standard Error 0.439033786 Standard
Error 0.405434832
Median 16 Median 17
Mode 17 Mode 17
Standard Deviation 3.673220193 Standard
Deviation 3.392111174
Sample Variance 13.49254658 Sample
Variance 11.50641822
Kurtosis 0.186350284 Kurtosis -0.313488844
Skewness -0.038801694 Skewness 0.403894173
Range 16 Range 15
Minimum 8 Minimum 10
Maximum 24 Maximum 25
Sum 1121 Sum 1178
Count 70 Count 70
Newman-Keuls
test; variable log total abundance. Probabilities for Post Hoc Tests Error:
Between MS = .02989, df = 252.00
|
|
Treatment
|
{1}
|
{2}
|
{3}
|
{4}
|
|
1
|
Empty
|
|
0.000010
|
0.312021
|
0.002005
|
|
2
|
Large
|
0.000010
|
|
0.000167
|
0.094946
|
|
3
|
Mixed
|
0.312021
|
0.000167
|
|
0.017216
|
|
4
|
Small
|
0.002005
|
0.094946
|
0.017216
|
|
Empty Small
Mean 46.88571429 Mean 62.12857143
Standard Error 3.034483857 Standard Error 4.979125013
Median 40 Median 51
Mode 28 Mode 44
Standard Deviation 25.38831345 Standard Deviation 41.65834865
Sample Variance 644.5664596 Sample Variance 1735.418012
Kurtosis 6.755817191 Kurtosis 8.744256028
Skewness 2.478854276 Skewness 2.694089527
Range 138 Range 227
Minimum 16 Minimum 16
Maximum 154 Maximum 243
Sum 3282 Sum 4349
Count 70 Count 70
Mixed Large
Mean 52.24285714 Mean 66.78571429
Standard Error 3.961326279 Standard Error 4.055050418
Median 46.5 Median 58.5
Mode 48 Mode 42
Standard Deviation 33.14283349 Standard Deviation 33.92698591
Sample Variance 1098.447412 Sample Variance 1151.040373
Kurtosis 11.73512173 Kurtosis 4.154559994
Skewness 2.916658065 Skewness 1.771172233
Range 205 Range 183
Minimum 16 Minimum 24
Maximum 221 Maximum 207
Sum 3657 Sum 4675
Count 70 Count 70
Newman-Keuls test; variable Species Richness. Probabilities for
Post Hoc
Tests Error: Between MS = 9.1321, df = 252.00
|
|
Treatment
|
{1}
|
{2}
|
{3}
|
{4}
|
|
1
|
Empty
|
|
0.000008
|
0.000022
|
0.000796
|
|
2
|
Large
|
0.000008
|
|
0.110918
|
0.000029
|
|
3
|
Mixed
|
0.000022
|
0.110918
|
|
0.001911
|
|
4
|
Small
|
0.000796
|
0.000029
|
0.001911
|
|
of a Nearshore
Artificial Reef in Broward County, Florida
Judy Robinson and Andrew
Rogerson
The
majority of artificial reef studies have focused on the interaction between
fish density and artificial physiography. Although colonization of artificial
reef structures has been studied to varying degrees (Gascon and Miller 1981;
Bonsack and Sutherland 1985; Haughton and Aiken 1989), relatively few studies
have quantitatively described the development of “attached” microbial or algal assemblages on artificial reef
structures, i.e. Reef Balls™.
Development
of microbial and algal films is important because they directly occupy the
substratum and provide secondary biotic space in the form of “lower story”
habitats (Dayton 1971). The physical
nature of the substratum has been shown to be an influence on substrate
selection by marine organisms in the laboratory (Crisp and Ryland 1960; Leitz
and Wagner 1993) and in the field (Harriot and Fisk 1987; Todd and Keough 1994;
Stoner 1994). The micro-scale features of substrate surfaces can differ in
surface tension, hydrophobicity, and surface texture (Mihm and Banta 1981).
Moreover, the nature to the microbial films could make an unattractive
substratum attractive. These films may act as a physical presence between
settled larvae and the immersed surface possibly enhancing adhesion. Crisp et al. (1985) reported that cypris
larvae might not permanently attach to a surface in which their adhesive does
not bind strongly, suggesting that extracellular material from bacterial films
may mediate greater adhesion.
Additionally,
chemosensory recognition of microbial films (Kirchman et al. 1982)
and of some algal species (Morse et al. 1994), are thought to play a role
in inducing settlement and metamorphosis of a wide variety of marine
invertebrate larvae, at least in the early stages of substratum colonization or
succession. Zobell and Allen (1935) first documented the importance of
microbial biofilms in the development of marine invertebrate communities. Since
their early work, a variety of marine organisms have been reported to display
varying responses to filmed and unfilmed surfaces. Brancato & Wollacott
(1982) observed that three species of bryozoans selected “filmed” in preference
to “non-filmed” substrata when given a choice. Moreover, when not offered a
choice, Bugula simplex and Bugula turrita preferred not to settle
on a non-filmed surface, whereas Buglua
stolonifera settled on filmed as well as non-filmed surfaces. However, it
is worth noting that available data concerning the necessity of primary organic
and micro-floral films for triggering larval settlement are highly variable.
Crisp and Ryland (1960) showed that biofilms were not an essential prerequisite
for larval settlement, while Kirchman et
al. (1982) demonstrated that a spirobin polychaete settled in response to
multi-species microbial films. Maki et al.
(1988, 1992) noted age-related effects both for larva and bacterial-organic
films. However, these experimental designs did not permit the assessment of the
respective contribution of initial substratum surface chemistry and the
influence of previously settled conspecifics on subsequent settlement.
Considerable emphasis has been placed
on the importance of supply and settlement of marine larvae in relation to
patterns of distribution and abundance of juveniles and adults (Underwood and
Fairweather 1989; Gleason 1996; Smith 1997). The events, factors, and processes
affecting larval settlement are evidently pivotal to the further development of
a given assemblage. An understanding of settlement is, therefore, an essential
pre-requisite to an appreciation of the overall population and assemblage
dynamics. The nature of the mechanisms inducing or inhibiting settlement is of
much interest, yet there are insufficient field data for a wide range of taxa.
Given the biological significance of these micro-scale communities to overall
reef productivity, we examined the first steps of fouling by microbial and
algal biota during the initial immersion of Reef Ball substrates.
Methods
Study Area:

260
03.382 N
800
05.746 W
|
|
Memphis
Project
Study Site
|
|
 The
study site is located in Broward County 0.5km offshore of the Hollywood Beach
area. This location is situated at the grounding site of the USS MEMPHIS
nuclear submarine that ran aground in
The
study site is located in Broward County 0.5km offshore of the Hollywood Beach
area. This location is situated at the grounding site of the USS MEMPHIS
nuclear submarine that ran aground in
 30
ft of water on a coral reef off
30
ft of water on a coral reef off
southeast Florida. Biofilm plate
arrays were
deployed on four
Reef Balls
(Quad) in the north-
east corner of
the Memphis
project site
(figure1).
Figure 1. Map of study site.
Experimental Design, Collection
and Preparation of Samples
The design of the experiment
involved four biofilm plate treatments (concrete only, concrete with CaCo3
surface coating, iron powder surface coating, and glass slides) placed in three
arrays, each consisting of 24 biofilm plates (figure 2a, one treatment per
array) and one array of 24 glass slides (figure 2b). One treatment array was
attached to a Reef Ball within the Reef Ball quad. Three biofilm plates were
collected from each treatment array at a given time interval (24h, 3 d, 7 d, 14
d, 21 d and 60 d).

 Biofilm plates were 15 mm in
diameter and constructed of the same concrete as the Reef Balls (figure
2c). Plates were soaked in distilled water for 2 weeks prior to deployment.
Biofilm plates were 15 mm in
diameter and constructed of the same concrete as the Reef Balls (figure
2c). Plates were soaked in distilled water for 2 weeks prior to deployment.
For examination of
the biofilm, 3.53 cm2 of the plate
surface and 9.37 cm2 of
the glass slide surface were scraped to
remove
and mixed with 2 mm filtered seawater. The microbiota
 were fixed in 2% glutaraldehyde and
then stained with the
were fixed in 2% glutaraldehyde and
then stained with the
DNA specific flourochrome DAPI.
Bacteria, algae, and other
flora and fauna were enumerated by
epifluorescence
microscopy  using UV illumination. In all cases, 50 random
using UV illumination. In all cases, 50 random
fields of view were counted per
samples.
Statistical Analysis
based on cell count data, treatment
effect was judged present
if the
F-statistic showed significance at α £ 0.05. If significant,
then Tukey’s HSD determined which treatments differed (α < 0.05).
Scatter plot and regression were fitted to pooled bacterial data summed across
replicates within three substrate treatments. Nonparametric procedures (c2
approximation of Kruskal-Wallis P£ 0.05) were employed in lieu of parametric ANOVA when
population variances were heterogeneous. Data analysis and graphing was
completed using SPSS 11.0 â software.
Results
Bacteria
and diatoms were usually the most abundant organisms found on the biofilm
plates. Significantly greater coverage of bacteria, diatoms and other
associated flora and fauna was seen on plates that did not contain the iron
surface treatment.
No
significant differences in bacterial densities were found between treatments in
most sampling intervals except for iron (figures 3-8, Tukey’s HSD α =
<0.05). Highest rates of settlement were on concrete and CaCo3
substrates at 14 and 21 days (figures 5 and 6, Tukey’s HSD α < 0.05).
Bacterial densities increased through time in three treatments, concrete, CaCo3,
and glass slides, with pooled mean densities reaching > 800,000 cells cm-2
and remaining somewhat stationary after 14 days (figures 9 and 10). However,
bacterial densities in iron treatments did not follow the same pattern as the
other treatments and bacterial colonizers remained low compared to the other
substrates (means £
16,600 cells cm-2, figures 1-8, and 11). Settlement was poorest on iron coated concrete in all trials.
Diatom
populations fluctuated (figures 12-17) throughout the study with peak density
at >80,000 cells cm-2 found on the concrete substrate at 21 days
(figure 16). Yet, no significant difference existed between substrate types at
60 days except for iron. At day 60, mean densities had dropped to < 40,000
cells cm-2 (figure 17) in three treatments; no diatoms were reported
in any iron treatments. Other autotrophic organisms also varied in numbers
(figures 18-23) but day 60 showed no difference between substrate types (figure
23, c2
α =.102).
Early
secondary micro-colonizers were mainly composed of known (i.e. nematodes and
flagellates) and unidentified heterotrophs. The density of these consumers
differed significantly between treatments over time (c2, α =
.000, .050, .011, and .012) with treatments of concrete, CaCo3, and
glass slides exhibiting the greatest densities (figures 24-29), ranging from
< 4000 cells cm-2 at 24 hours to >14,000 cm-2 at 60
days. The number of heterotrophic fauna remained very low on the iron substrate
(figures 24-29) and after three days none were found (figures 26-29). Copepods,
small molluscs, and small polychaetes comprised the remaining group of fauna
associated with the majority of the biofilm plates.
Discussion
Microbial
and algal films have been indicated as probable settlement inducers of marine
benthic invertebrates, in addition, to providing secondary biotic space and
shelter. Marine larvae are capable of responding to a variety of
physico-chemical cues, some which may originate from organic films, during
substratum exploration (Johnson et al.
1991; Anderson 1996; Johnson and Sutton 1994 and Raimondi and Morse 2000).
Larvae may respond to biofilms because they may reflect the physical regime
that has acted on that surface over time (Wiecsorek and Todd 1998), relaying
information about surface suitability for larval settlement and post-settlement
survival. Therefore, the period of immersion and subsequent biofilm formation
may be of paramount importance when assessing the colonization of secondary fauna
on these artificial reef structures.
Results for three of the four
treatments were not dramatically influenced by substrate type and bacterial
numbers were similar. The single discrepancy in results was at day 60 where one
replicate had an exceedingly high count of 2,410,847 cells cm-2.
This suggests that even when organic films are developed over the same period
under the same conditions, film formation can be patchy and cause important
differences in colonization. Previous
preliminary results (Robinson and Rogerson 2001) also demonstrated that
bacteria and diatoms colonized surfaces of Reef Ball concrete and the count per
unit appeared to increase with time (densities ranging between > 50,000
cells cm-2 to < 120,000 cm-2 and > 2500 cm-2 at
28 days, respectively). The number of bacterial colonizers were comparable to
film formation on three of the four substrates in this study.
Colonization
was uniformly low in all trails with iron surface coating. We hypothesize that
initial substrate conditions may not have been attractive to microbial or
micro- colonizers, in part, due to the potential interaction with the harsh
environment, which may have effected biofilm formation and attachment
preferences. Because iron concentrations on the substrate surface were
extremely high and choice of this substrate for settlement was low, detailed
interpretation of these results should be regarded with caution.
Microbial and
algal films, which are ubiquitous in the marine environment, are probably not
substratum specific, although some selectivity in extreme environments is
likely (i.e. heavy metals, Basalobre 1993). Substrate type may be much less
important in controlling the distribution and abundance of microbial colonizers
than factors or events occurring after settlement, such as predation, disease,
or migration. Our results revealed that, with the exception of iron coated
concrete substrate, the development of the microbial film on the concrete
substrates (concrete alone, concrete with CaCo3) proceeded at a rate
comparable to the control material (glass). The precise role of biofilms in
larval settlement remains unknown, but it is encouraging that the material used
in the Reef Ball structures does not noticeably affect film formation.
F=
[3,8] =6.712*
a = .014
|
|
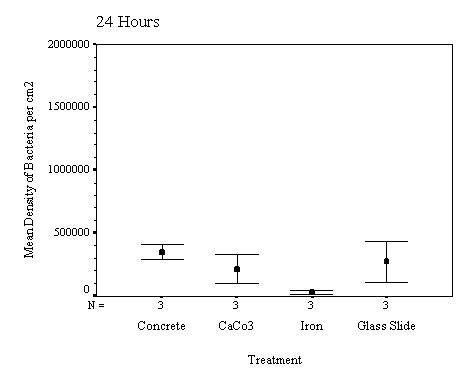
Figure 3.
F=
[3,8] =12.69*
a = .084
F=12.169*
|
|
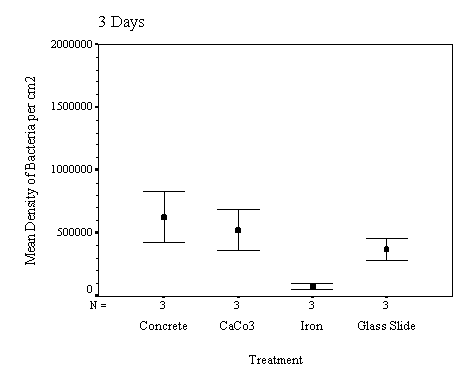
Figure 4.
Figures 3 and 4. Settlement responses of bacteria on
the four substrates.
*Significant at P≤
0.05, ANOVA. Lower case letters identify treatments not significantly different
(Tukey’s HSD P<0.05). Error bars show mean +/- 2.0 SE.
F=
[3,8] =17.083*
a =.001
F=17.083*
|
|
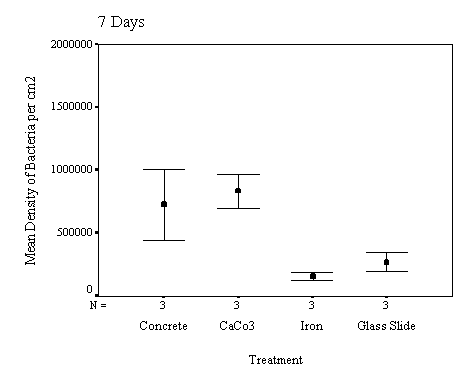
Figure 5.
F=
[3,8] =16.270*
a =.099 a
a
|
|
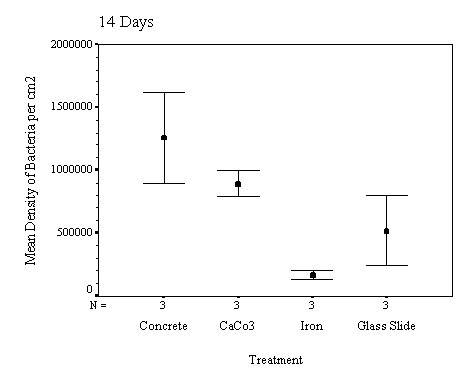
Figure
6.
Figures 5 and 6. Settlement responses of bacteria on
the four substrates.
*Significant at P≤ 0.05, ANOVA. Lower case
letters identify treatments not significantly different (Tukey’s HSD
P<0.05). Error bars show mean +/- 2.0 SE.
F=
[3,8]=13.524*
a =.002
=13.524*
|
|
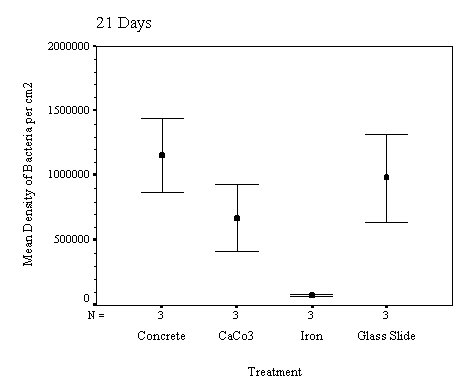
Figure 7.
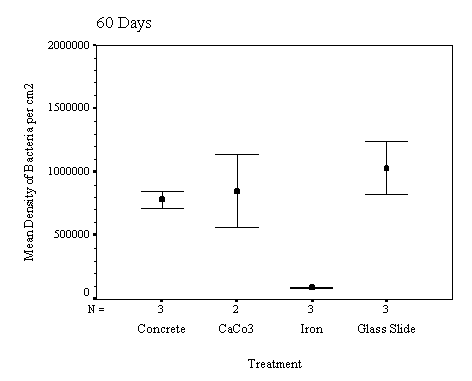
Figure 8. -- Figures 7 and 8.
Settlement responses of bacteria on the four substrates.
*Significant at P≤ 0.05, ANOVA. Lower case
letters identify treatments not significantly different (Tukey’s HSD
P<0.05). Error bars show mean +/- 2.0 SE. Replicate #3 at day 60 was
excluded from the analysis.
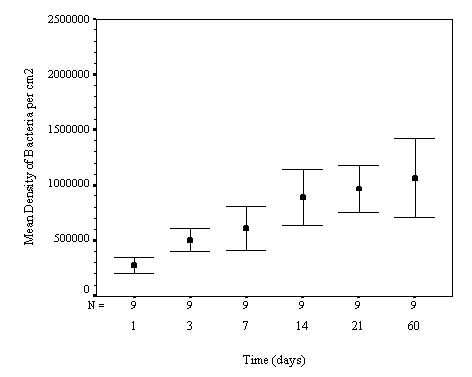
Figure 9. Mean densities of pooled bacterial counts
over time: treatments concrete, CaCo3, and glass slide. Error bars show mean +/- 2.0
SE.
1
3 7 14
21
60
Time
(days)
Figure 10. Relationship
of pooled bacterial densities on concrete, CaCo3, and glass
slides treatments from initial deployment to day 60 (n=36).
|
|
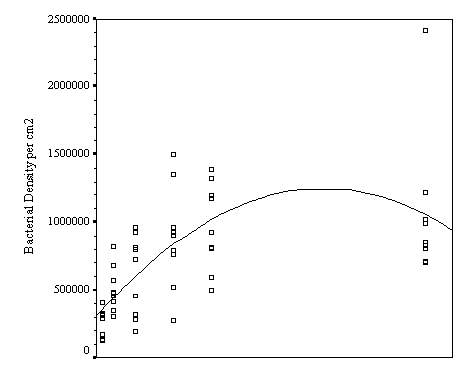
1
3 7 14 21
60
Time (days)
|
|
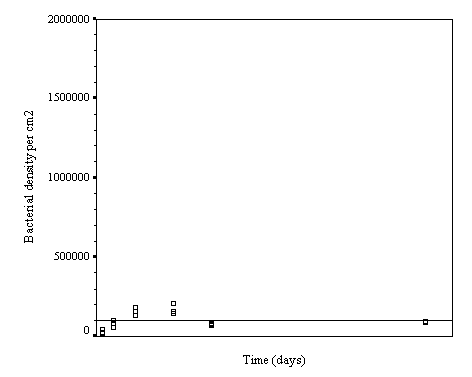
Figure
11. Relationship of bacterial densities on iron treatment from initial
deployment to day 60 (n=18).
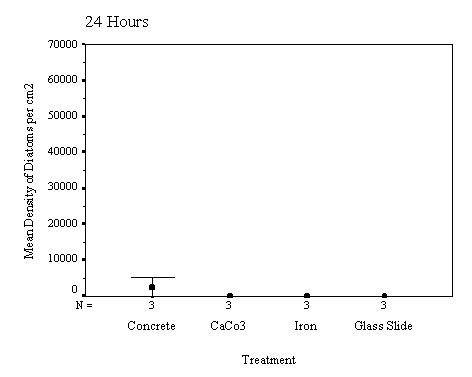
Figure 12.
F=
[3,8] =.966
a =.443
F=
.966
|
|
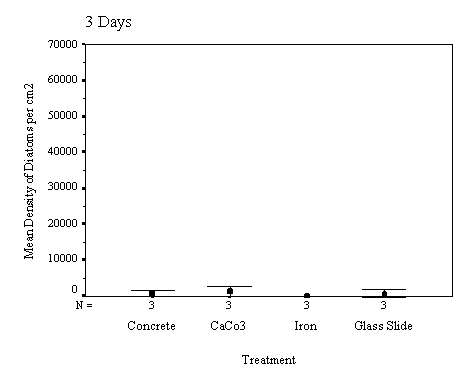
Figure 13.
Figures
12 and 13. Settlement responses of diatoms on the four substrates.
*Significant at P≤ 0.05, ANOVA. Lower case
letters identify treatments not significantly different (Tukey’s HSD
P<0.05). Error bars show mean +/- 2.0 SE. No analysis was performed on 24
hour treatments.
F=
[3,8] =46.135*
a =.000
F=
46.135*
|
|
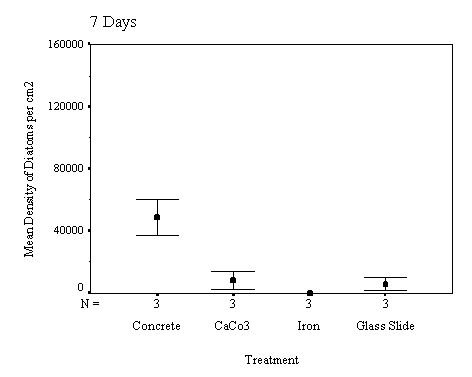
Figure 14.
F=
[3,8] =3.415
a =.123
F=
3.415
|
|
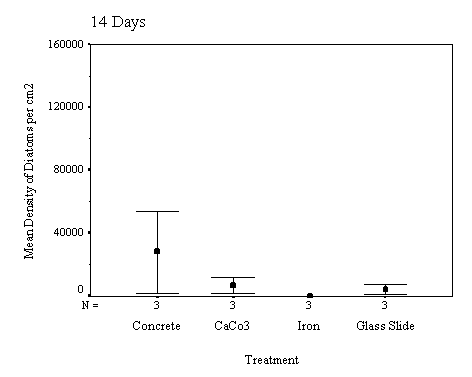
Figure 15.
Figures
14 and 15. Settlement responses of diatoms on the four substrates.
*Significant
at P≤ 0.05, ANOVA. Lower case letters identify treatments not
significantly different (Tukey’s HSD P<0.05). Error bars show mean +/- 2.0
SE.
F=
[3,8] =21.963*
a =.000
|
|
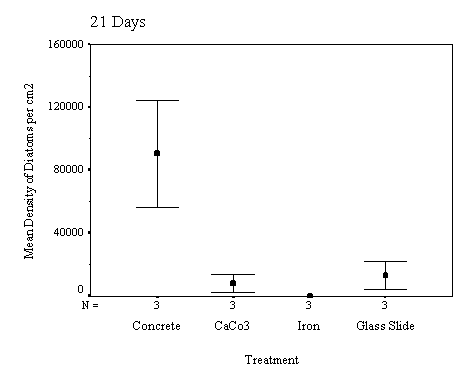
Figure 16.
F=
[3,8] = 1.988
a =.194
F=
1.988
|
|
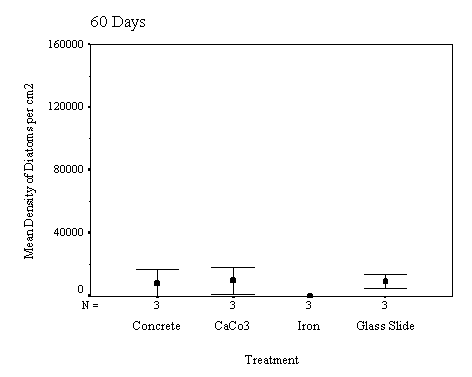
Figure 17.
Figures
16 and 17. Settlement responses of diatoms on the four substrates.
*Significant
at P≤ 0.05, ANOVA. Lower case letters identify treatments not
significantly different (Tukey’s HSD P<0.05). Error bars show mean +/- 2.0
SE
References
Anderson, M. 1996. A chemical cue induces settlement
of Sydney Rock oysters, Saccostrea
commercialis, in the laboratory and in the field. Biol. Bull. 190: 350-358.
Balsalobre, C., Calonge, J., Jimenez, E., Lafuente,
R., Mourino, M., Munoz, M., Rquelme, M., and Mas-Catella, J. 1993. Using the
metabolic capacity of Rodaobacter
sphaeroides to assess heavy metal toxicity. Environ. Toxicol. Water Qual.
8: 437- 450.
Brancato, M., and R. Woollacott. 1982. Effect of
microbial films on settlement of
bryozoan larvae (Bugula simplex,
B. stolonifera, and B. turrila). Mar. Bio. 71: 551-56.
Bonsack, J., and Sutherland, D.
1985. Artificial reef research: a review with recommendations for future
priorities. Bull. Mar. Sci. 37: 11-39.
Crisp, D., and J. Ryland. 1960. Influence of filming and the
surface texture on the settlement of marine organisms. Nature 185: 119.
Crisp, D., G. Walker, A. Young, and A.
Yule. 1985. Adhesion and substrate choice in mussels and barnacles. J. Colloid Interface Sci. 104:
40-50.
Gleason, M. 1996. Coral recruitment in Moorea, French
Polynesia: the importance of patch type and temporal variation. J. Exp. Mar.
Bio. And Eco. 207: 79-101.
Harriot, V., and D. Fisk. 1987. A comparison of settlement
plate types for experiments on the recruitment of scleractinian corals. Mar.
Eco. Pro. Ser. 37: 201-208.
Johnson, C., D. Muir, and A. Reysenbach. 1991. Characteristic
bacteria associated with surfaces of coralline algae: a hypothesis for
bacterial induction of marine invertebrate larvae. Mar. Ecol. Prog. Ser. 74:
281-294.
Johnson, C. and D. Sutton. 1994. Bacteria on the surface of
crustose coralline algae induce metamorphosis of the crown-of-thorns starfish Acanthaster planci. Mar. Bio. 120:
302-310.
Keough, M., and P. Raimondi. 1995. Responses of settling
invertebrate larvae to bioorganic films: effects of different types of films.
J. Exp. Mar. Bio. Eco. 185: 235-253.
Kirchman, D., D. Reish, and R. Mitchell. 1982. Bacteria
induced settlement and metamorphosis of Janua
(Dexiospira) brasiliensis Grube (Polychaeta: Spirorbidae. J. Exp. Mar. Bio.
Eco. 56: 153-163.
Leitz, T., and T. Wagner. 1993. The marine bacterium Alteromonas espejiana induces
metamorphosis of the Hydractinia echinata.
Mar. Bio. 115: 173-178.
Maki, J., D. Rittschof, J. Costlow, and R. Mitchell.
1988. Inhibition of attachment of larval barnacles, Balanus amphirite, by bacterial surface films. Mar. Bio. 97:
199-206.
Maki, J., D. Rittschof, and R. Mitchell. Inhibition of larval
barnacle attachment to bacterial films: an investigation of physical
properties. Microbial. Eco. 23: 97-106.
Mihm, J., and W. Banta. 1981. Effects of adsorbed organic and
primary fouling films on bryozoan settlement. J. Exp. Mar. Bio. Eco. 54:
167-179
Morse, A., C. Froyd, and D. Morse. 1984. Molecules from
cyanobacteria and red algae that induce larval settlement and metamorphosis in
the mollusc Haliotis rufescens. Mar.
Bio. 81: 293-298.
Morse, D., A. Morse, P. Raimondi, and N. Hooker. 1994.
Morphogen-based chemical flypaper for Agaria
humilis coral larvae. Biol. Bull. 186: 172-181.
Smith, S. 1997. Patterns of coral settlement,
recruitment, and juvenile mortality with depth at Conch Reef, Florida. Proc. 8th
Int. Coral Reef Sym. 2: 1197-1202.
Raimondi, P. and Morse, A. 2000. The consequences of complex larval
behavior in a coral. Ecology 8: 3193-3211.
Robinson, J. and A. Rogerson. 2001. Preliminary analysis of
the initial microfouling communities of a nearshore artificial reef in Broward
County, Florida. American Society of Limnology and Oceanography conference.
Feb. 2001
Stoner, D. 1994. Larvae of a colonial ascidian use a
non-contact mode of substratum selection on a coral reef. Mar. Bio.
121:319-326.
Todd, C., and M. Keough. 1994. Larval settlement in hard
substratum epifaunal assemblages: a manipulative field study of the effects of
substratum filming and the presence of incumbents. J. Exp. Mar. Bio. Eco. 181:
159-187.
Underwood, A., and P. Fairweather. 1989. Supply-side ecology
and benthic marine assemblages. Trends Ecol. & Evol. 4: 16-20.
Wieczorek, S. and C. Todd. 1998. Inhibition and facilitation
of settlement of epifaunal marine invertebrate larvae by microbial biofilm
cues. Biofouling, 12: 18-188.
Zobell, C., and E. Allen. 1935. The significance of marine
bacteria in the fouling of submerged surfaces. J. Bacteriol. 29: 239-251.
1) Restoration Of
A Southeast Florida USA Coral Reef Injured By The Grounding Of A Nuclear
Submarine. Coral Reefs. Dodge R.E., R.E. Spieler, D.S. Gilliam, P. Quinn, A.
Rogerson, E. Glynn, K. Banks, L. Fisher, D. Stout and W. Jap. 2000.
2) Preliminary Analysis of Initial Microfouling of a
Nearshore Artificial Reef in Broward
County, Florida. American Society for Limnology and Ocanorgraphy. Judy
Robinson and Andrew Rogerson. 2001.
3)
Hypothesis-based Restoration Study for Mitigation of a Damaged S.E. Florida
Coral Reef: A Work in Progress.
Artificial Reef Summit. T.P. Quinn, E.A. Glynn, R.E. Dodge, K. Banks , L.
Fisher, R.E. Spieler
4) Growth and survivorship of stony coral Meandrina meandrites and Montastrea cavernosa transplants to an
artificial reef environment. Artificial Reef Summit Glynn EA, Quinn TP, Fahy
DP, Dodge RE, Spieler RE, and Gilliam DS.
5) Growth And
Survivorship Of Scleractinian Coral Transplants And Effectiveness Of
Plugging Core Hole Sites. International Society for Reef Studies. E.A.
Glynn, T.P. Quinn, D.P. Fahy, and R.E. Spieler. 2002.
Abstract
Presented:
Florida Artificial Reef Summit 2001, Ft Lauderdale,
FL xxxxxxxxxxxx
Growth and
survivorship of stony coral Meandrina
meandrites and Montastrea cavernosa transplants
to an artificial reef environment.
Glynn
EA, Quinn TP, Fahy DP, Dodge RE, Spieler RE, and Gilliam DS. National Coral Reef Institute (NCRI). Nova Southeastern University Oceanographic
Center. 8000 North Ocean Drive, Dania
Beach, FL 33004
In November of 2000, 160 Reef BallTM concrete modules (1.22m wide x 0.9m high) were deployed, at
a depth of approximately 15 meters, between the Second and Third Reef tracts off
Dania Beach, FL. Eighty, four-inch
diameter core plugs containing living tissue from 20 specimens of each of two
species of scleractinian corals Meandrina
meandrites and Montastrea cavernosa were
transplanted to the Reef Balls
between
March and June, 2001.
The coral core
plug transplants were obtained from coral colonies at approximately 10 meter
depth located on the Second Reef, off Dania Beach, using a hydraulic drill
fitted with a 4” diameter core barrel.
Forty of the 160 Reef Balls were designated as ‘coral transplant’ Reef
Balls. Each of these Reef Balls was constructed with two depressions, into which the two
different species of transplants were attached using an underwater adhesive
marine epoxy.
The drill
holes in the donor corals were filled with concrete plugs to prevent
detrimental effects of bioeroders. The
core hole sites are being monitored for tissue growth surrounding the concrete
plug. The transplant corals, donor
corals, and control corals of comparable size to both the large donor colonies
and the small transplant corals are being monitored for growth and
survivorship.
Coral skeletal
growth is defined as an increase in surface area or linear radius and is being
measured quarterly using photographic techniques. The large donor corals and comparable controls are being
photographed using a Nikonos V camera with 20mm lens and a 0.75m2
PVC framer marked in 10cm increments.
The coral plug transplants and comparable controls, and the core hole
sites are being photographed with a 28mm lens and close up kit. SigmaScan Pro4 image analysis software
(Jandel Scientific Corporation) is being used for the photographic
analysis. This monitoring method is
suitable for continuous monitoring and causes no harm to the coral colony.
The preliminary results obtained during the first quarterly sample
session demonstrated 100% survivorship for all coral plug transplants and donor
colonies. It was noted that most coral
plug transplants, with exposed skeleton around the margins of the plug, experienced
tissue advancement over the bare coral skeleton.
Abstract Presented:
International
Society for Reef Studies, Cambridge UK, Sept.
2002
GROWTH AND SURVIVORSHIP OF SCLERACTINIAN CORAL TRANSPLANTS
AND EFFECTIVENESS OF PLUGGING CORE HOLE SITES.
E.A. Glynn, T.P. Quinn, D.P. Fahy, and
R.E. Spieler
National Coral Reef Institute, Nova
Southeastern University Oceanographic Center
8000 North Ocean Drive, Dania Beach, FL
USA 33004. email: glynn@nova.edu
Eighty core plugs containing living tissue (coral transplants) of two
species of scleractinian coral Meandrina
meandrites (n=40) and Montastrea cavernosa (n=40) were transplanted to forty Reef BallTM
modules between March and June, 2001.
The cores were obtained from forty individual coral colonies, on an
adjacent natural reef, using a hydraulic drill fitted with a four-inch core
barrel. Two cores were sampled from
each of the forty donor colonies. All
donor core holes were filled with pre-fabricated, numbered concrete plugs to
prevent the detrimental effects of bioeroders.
Core hole sites and transplant corals, as well as control corals of
comparable size (to both the large donor colonies and the small transplant
corals), were monitored for growth and survivorship. Coral skeletal growth has been defined as an increase in surface
area or linear radius and has been measured quarterly using photographic
techniques. The large donor corals and
comparable controls were photographed using a Nikonos V camera with 20mm lens
and a 0.75m2 PVC framer marked in 10cm increments. The core hole sites, coral plug transplants
and comparable controls were photographed with a 28mm lens and close up
kit. SigmaScan Pro4 image analysis
software (Jandel Scientific Corporation) was used for the photographic
analysis. This monitoring method is suitable for continuous monitoring and
causes no apparent harm to the coral colony.
After nine months of sampling, 100% of the M. cavernosa and 71% of the M.
meandrites transplants maintained their original tissue surface area or
showed evidence of an increase in surface area. The remaining 29% of the M.
meandrites transplants have shown varying degrees of partial tissue
mortality.
The donor colonies have experienced
100% colony survival. The core hole sites have not regenerated tissue over the
concrete plugs. There has been little tissue die back from the plug sites and
so regeneration remains possible. Although it is too early in the study to draw
firm conclusions, the species specific differences in transplant growth and
mortality may be an important consideration in future coral reef restoration
efforts.
Figure 3: Map of second reef depicting
site of donor (D) and control (C) corals (note area of USS Memphis
grounding trench and DPEP damage control pins: CP1, 2, & 3).
|
|