Go back to Chapter 4 Go back to Table of Contents
Next: References
ABSTRACT
Laboratory concretes containing only coarse aggregates and 0, 10, or 20% silica fume as a cement replacement were prepared at a constant water-to-solids ratio and sampled at 1, 7, and 28 days. Scanning electron microscopy was utilized to monitor the progress of the hydration reactions both in the bulk paste and in regions near an aggregate surface. Phase volume fractions were determined by quantitative image analysis of the backscattered electron images. In addition, the size of "individual" two-dimensional cross sections of calcium hydroxide crystals and capillary pores were assessed. Silica fume additions are seen to affect both the amount and size of these microstructural features. Differences are observed between interfacial zone and bulk areas which support previous mercury intrusion measurements of mortar specimens. This analysis lends support to the hypothesis that when silica fume is present, calcium hydroxide crystals form and then dissolve away, contributing to a connected pathway of capillary porosity in the interfacial zone regions.
Introduction
In recent years, condensed silica fume (CSF) has found wide usage in producing high performance concretes. Silica fume improves concrete properties in two ways: its small particle size results in a filler effect in which the silica fume particles bridge the spaces between cement grains and the spaces between cement grains and aggregates; and, the silica fume reacts pozzolanically with calcium hydroxide to produce a greater solids volume of calcium silicate hydrate gel, leading to an additional reduction in capillary porosity during hydration [1]. While silica fume has little effect on the strength of cement pastes, it generally markedly increases the strength of concrete [1]. This has been attributed to the production of a denser and more homogoneous interfacial zone as documented by scanning electron microscopy (SEM) [2,3,4] and microhardness [1] measurements.
Although the interfacial zones formed in the presence of silica fume are denser, several researchers have obtained mercury intrusion porosimetry results which suggest the formation of a network of coarse pores in mortars and concretes containing silica fume [5,6,7]. It has been suggested that the presence of these pores is due to the dissolution of calcium hydroxide crystals during the pozzolanic reaction which occurs topochemically at the silica fume surfaces. Indirect evidence for this phenomena has been provided by Buil and Delage [8], who reacted calcium hydroxide crystals and silica fume particles in water and observed the replicates of calcium hydroxide crystals present as voids in the final paste. Similar replicas of calcium hydroxide crystals have been observed in pastes containing fly ash [9]. The goal of the present research is to provide more direct evidence for this phenomena by monitoring the volume fraction of calcium hydroxide and size of calcium hydroxide crystals and capillary pores as a function of hydration for concretes containing 0, 10, and 20% silica fume.
Experimental Procedure
Sample Preparation
Sample preparation is described in detail elsewhere [3]. All three concrete mixes were prepared at a water/solids (w/s) ratio of 0.45 with mix 1 containing no silica fume and mixes 2 and 3 having 10 and 20% respectively mass replacement of the cement by silica fume. A quartz river gravel (-1/2 to + 1/4 mesh) was used as aggregate in all mixes in a 1:1 proportion by mass to the cementitious material (cement and silica fume).
The mixing procedure consisted of premixing the silica fume (when present) with the water, adding the cement and mixing until wet, and then adding the aggregate in preparation for final blending. Final mixing proceeded for three minutes, was halted for three minutes while the mixing bowl walls were scraped down, and resumed for a second period of three minutes. The concrete was cast in 2.5 cm steel cube molds and was vibrated for 10 seconds on a shaker table to aid in consolidation. All cubes were demolded after 24 hours with some being set aside to comprise the 1 day samples. The remainder were placed in saturated lime (calcium hydroxide) water and cured until sampled at 7 and 28 days.
Quantitative Image Analysis
To prepare the samples for viewing with the SEM, the sampled specimens were placed in dried ethanol to replace the pore solution and then in an ultra-low viscosity epoxy embedding resin to replace the ethanol [10]. This procedure allows the preparation of a polished section without drying, thus minimizing the occurrence of drying cracks. The resin was cured and the samples were ground with a series of 6, 3, 1, and 0.25 Ám diamond pastes on a low-speed lap wheel using propylene glycol as a polishing lubricant. A 100 nm thick coating of carbon was evaporated onto the polished surface to eliminate specimen charging in the SEM.
In the SEM, an image of the microstructure is collected using the
backscattered electron (BE) detector. In the BE image, contrast is dependent on
the average atomic number (![]() ) of a phase, with higher
) of a phase, with higher ![]() phases appearing brighter. For hydrated cement, the major phases
from brightest to darkest are anhydrous cement, calcium hydroxide, calcium
silicate hydrate gel, and resin-filled porosity. Because these brightnesses are
fairly distinct, the BE images can be analyzed to determine quantitative phase
abundance data. Image analysis techniques have been applied to characterizing
the microstructure of cement pastes and concrete [11,12,13] and more
recently to distinguishing the various clinker phases in unhydrated Portland
cement powder [14]. To
discriminate phases, the greylevel histogram is calculated and analyzed to
determine the greylevel range occupied by each phase of interest. The greylevel
histogram is simply a histogram of the number of pixels (image elements) present
in an image for each greylevel from 0 (darkest) to 255 (brightest) [15].
Ideally, each phase will show up as a separate peak in the greylevel histogram.
In this case, discrimination will be a trivial task, as the minima between each
pair of adjoining peaks provides a point for separating the two phases [15].
Oftentimes, however, two peaks will overlap one another and the midpoint must be
determined by other techniques such as the tangent slope technique described by
Scrivener et al. [13].
phases appearing brighter. For hydrated cement, the major phases
from brightest to darkest are anhydrous cement, calcium hydroxide, calcium
silicate hydrate gel, and resin-filled porosity. Because these brightnesses are
fairly distinct, the BE images can be analyzed to determine quantitative phase
abundance data. Image analysis techniques have been applied to characterizing
the microstructure of cement pastes and concrete [11,12,13] and more
recently to distinguishing the various clinker phases in unhydrated Portland
cement powder [14]. To
discriminate phases, the greylevel histogram is calculated and analyzed to
determine the greylevel range occupied by each phase of interest. The greylevel
histogram is simply a histogram of the number of pixels (image elements) present
in an image for each greylevel from 0 (darkest) to 255 (brightest) [15].
Ideally, each phase will show up as a separate peak in the greylevel histogram.
In this case, discrimination will be a trivial task, as the minima between each
pair of adjoining peaks provides a point for separating the two phases [15].
Oftentimes, however, two peaks will overlap one another and the midpoint must be
determined by other techniques such as the tangent slope technique described by
Scrivener et al. [13].
Specifically, for the systems analyzed here, five images each of bulk paste and interfacial zone regions were analyzed for each silica fume content and age, for a total of 90 images. The images were taken at a resolution such that each of the 512 x 398 pixels in an image was 0.4 Ám on a side. In general, for a given age and silica fume content, the threshold values (i.e. the values used to specify the greylevel range of a phase) could be maintained constant for all five images. Additionally, these values were typically determined by selecting the minima between adjoining peaks which were generally quite distinct. Based on the threshold values selected, the degree of hydration of the cement in the bulk paste images could be determined as one minus the ratio of the fraction anhydrous cement in the image to the fraction anhydrous cement that would have been present initially for a w/s ratio of 0.45 and the appropriate silica fume content. The area fraction of calcium hydroxide could be determined as the number of calcium hydroxide pixels divided by the total number of pixels in the image (neglecting the aggregate). The size of each two-dimensional pore and calcium hydroxide crystal was also determined in order to follow the behavior of the larger crystals and pores as a function of time and silica fume content.
Results and Discussion
Representative microstructures from this series of specimens have been presented previously [3], so only the results of the quantitative analyses will be given here. Table I shows the average degree of hydration achieved in each set of images, along with the standard error for each image set in parentheses. Considerable hydration has already occurred in the one-day specimens. Because the pozzolanic reaction fills in porosity more efficiently than the formation of calcium hydroxide does, the ultimate degree of hydration achieved for the systems containing silica fume is less than that for the neat paste system. In fact, in terms of anhydrous cement, no substantial hydration is observed to occur between 7 and 28 days for the systems containing silica fume. This observation will be important in interpreting the changes in porosity and calcium hydroxide which occur in these systems between 7 and 28 days, as results presented later in this paper indicate that the pozzolanic reaction does continue during this time period.
Table I: Degree of Hydration vs. Time
Figure 1 shows the evolution of calcium hydroxide over time for the three different mixes. On average, the standard deviation for the calcium hydroxide area fraction for a set of five images was 0.013. In figure 1, for the system containing no silica fume, the calcium hydroxide content is seen to increase slightly over the course of the 28 days due to the continued hydration. Conversely, for the systems containing silica fume, as would be expected, a decrease in calcium hydroxide area fraction due to the pozzolanic reaction was observed. This decrease was more substantial for the system containing the higher volume fraction of silica fume. In general, for the calcium hydroxide area fraction, little difference is observed here between the interfacial zone and bulk paste images, with the bulk paste images containing slightly more calcium hydroxide than the interfacial zone images. This would be expected for the specimens containing silica fume, as initially, there will be a greater concentration of silica fume to participate in the pozzolanic reaction in the interfacial zone regions than in the bulk paste [3].
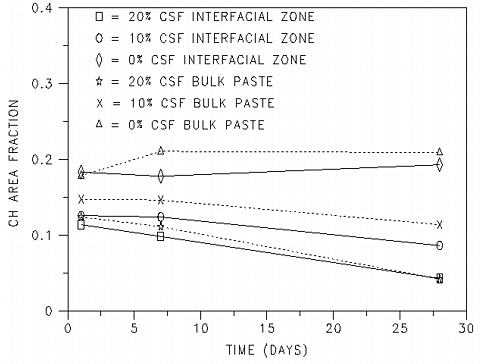
Figure 1: Calcium hydroxide area fraction vs.
time for samples containing 0, 10, and 20% silica fume.
The calcium hydroxide content vs. time data presented here is consistent with reported data for a series (0-25% silica fume replacement in 5% increments) of w/s=0.48 cement/silica fume paste systems [16] and a series (0, 8, and 16% silica fume replacement) of w/s=0.4 systems [17]. In both of these previous studies, the calcium hydroxide contents were determined on a mass basis by thermogravimetric analysis, in contrast to the image analysis procedures used here. For all three sets of results, silica fume contents in the range of 8-10% are seen to provide a relatively constant level of calcium hydroxide after one day of hydration. In contrast, systems containing 15-20% silica fume are seen to exhibit a slight decrease in calcium hydroxide content from 1 to 7 days with a more substantial decrease between 7 and 28 days.
In addition to the area fraction of calcium hydroxide, it is also of interest to monitor the size of the calcium hydroxide crystals. Here, the assumption is being made that the area of these crystals in two dimensions is indicative of their volume in three dimensions. To do this, the area occupied by crystals larger than 16 Ám2 (100 pixels) in area was determined as a function of time and silica fume content. The results, plotted in figure 2 indicate that the area occupied by "large" crystals is increasing with time in the 0% silica fume system. This would be expected as the hydration continues and as large crystals continue to grow at the expense of smaller ones due to Oswald ripening effects. In contrast, this value is seen to decrease in the systems with either 10% or 20% silica fume. For this to occur, some of the large crystals of calcium hydroxide must be either partially or totally dissolving to provide calcium ions for the pozzolanic reaction with the silica fume. If the pozzolanic reaction occurs away from the dissolution site, a pore will be created at the original dissolution site. While not as significant as the effects of silica fume addition, in general, there is a greater area occupied by large crystals in the bulk paste images than in the interfacial zone images, consistent with the higher overall area fractions of calcium hydroxide in the bulk paste regions.
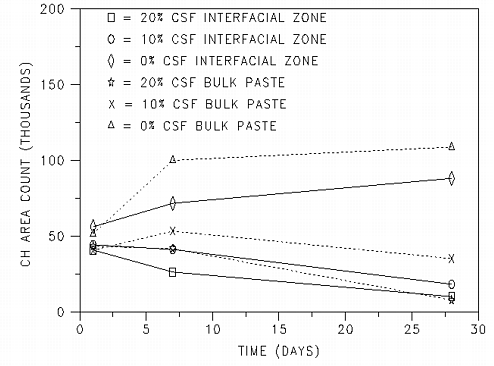
Figure 2: Area of calcium hydroxide crystals
larger than 16 Ám2 (100 pixels) vs. time for samples containing 0,
10, and 20% silica fume.
Further insight into the effects of the pozzolanic reaction can be obtained by monitoring the area occupied by large capillary pores as a function of age. Figure 3 provides a plot of the area occupied by pores larger than 16 Ám2 (100 pixels) vs. time. For the systems with 0% silica fume, the area occupied by these large pores decreases substantially between 1 and 28 days. The interfacial zone images contain a much greater area of large pores than the bulk paste images consistent with the generally higher porosity of interfacial zone regions observed previously [2,3]. For the systems containing silica fume, the results are quite different as the area occupied by large pores actually increases with increasing age for both sets of the interfacial zone images and for the bulk paste images for the system containing 20% silica fume. For the bulk paste images of the system containing 10% silica fume, the area occupied by the large pores is relatively constant over time, as was the area occupied by large crystals of calcium hydroxide. In general, the development of these large pores is more significant in interfacial zone regions than in the bulk paste. Furthermore, the appearance of these larger pores correlates directly with the disappearance of some of the larger crystals of calcium hydroxide.
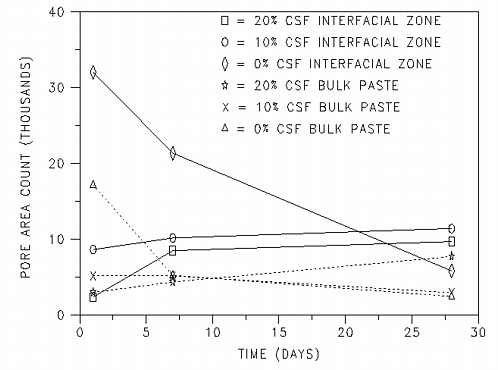
Figure 3: Area of pores larger than 16
Ám2 (100 pixels) vs. time for samples containing 0, 10, and 20%
silica fume.
These observations, when taken collectively, support the hypothesis that in cement-based systems containing silica fume, the pozzolanic reaction may result in the transformation of calcium hydroxide crystals into relatively large pores, predominantly in regions near an aggregate surface. Because the increases in the area occupied by large pores are occurring during times when little hydration of the anhydrous cement is observed, it is more likely that the pores forming at later ages (after 1 day) are due to the dissolution of previously formed calcium hydroxide crystals than to the formation of the hollow hydration shells commonly referred to as Hadley grains [18]. These direct SEM observations are supported by the mercury intrusion measurements of paste and mortar specimens made by Feldman [6] on specimens with 10% silica fume and w/s=0.6 and Winslow et al. [7] on specimens with 10% silica fume and w/s=0.4. Thus, even though the presence of silica fume greatly densifies the overall and interfacial zone microstructures, large pores may remain. If these pores are present in significant numbers and there are enough aggregates in a concrete that the interfacial zone regions percolate [7], these pores may themselves percolate across a concrete, providing a path less resistant to the ingress of deleterious ions such as chlorides or sulfates, as observed experimentally in mortar samples [19,20].
Figure 3 suggests that the presence of these pores is a function of silica fume content. For instance, silica fume contents less than 10% may actually be superior in this respect as the pozzolanic reaction may not occur to an extent that the larger crystals of calcium hydroxide are totally consumed. Likewise, for silica fume contents greater than 20% and a low enough w/s ratio, the pozzolanic reaction may occur to such an extent that virtually no capillary porosity (and no calcium hydroxide) is left in the sample [21]. Although not a parameter in this study, lower w/s ratios should also be beneficial as less calcium hydroxide will be produced [16,17] due to the lower overall degree of hydration which can be achieved in such systems. In fact, for a w/s=0.24 system containing 6% silica fume replacement, Sarkar and Aitcin [22] detected no evidence of large pores (> 0.1 Ám) via mercury intrusion porosimetry for a concrete cured for 91 days. It is expected that this phenomena will also be a function of water-to-solids ratio and curing history as both the pozzolanic reactivity of silica fume and the morphology of calcium hydroxide crystals have been shown to be functions of curing temperature [23]. Obviously, further evaluation is necessary to determine the implications and importance of these large pores in field concrete samples.
Conclusions
Quantitive phase analysis, based on scanning electron microscopy and image analysis, has been utilized to study the evolution of calcium hydroxide and porosity in "concrete" specimens containing only large aggregates. At a constant w/s ratio of 0.45, systems based on three silica fume contents (0, 10, and 20% cement replacement on a mass basis) have been studied. The trends measured for degree of hydration and area fractions of large calcium hydroxide crystals and pores suggest the phenomena of a transformation of calcium hydroxide crystals into porosity via dissolution during the pozzolanic reaction between calcium hydroxide and silica fume. This observation is consistent with a class of large pores detected previously using mercury intrusion porosimetry to study pastes and variable sand content mortars containing silica fume. As this study is preliminary in nature, further research is needed to assess the effects of mix parameters and curing conditions on the presence and consequences of these large interfacial zone pores.